Polycarbonate preparation method
A technology of polycarbonate and acid-binding agent, which is applied in the field of polycarbonate preparation to achieve the effects of mild reaction conditions, avoiding side reactions, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
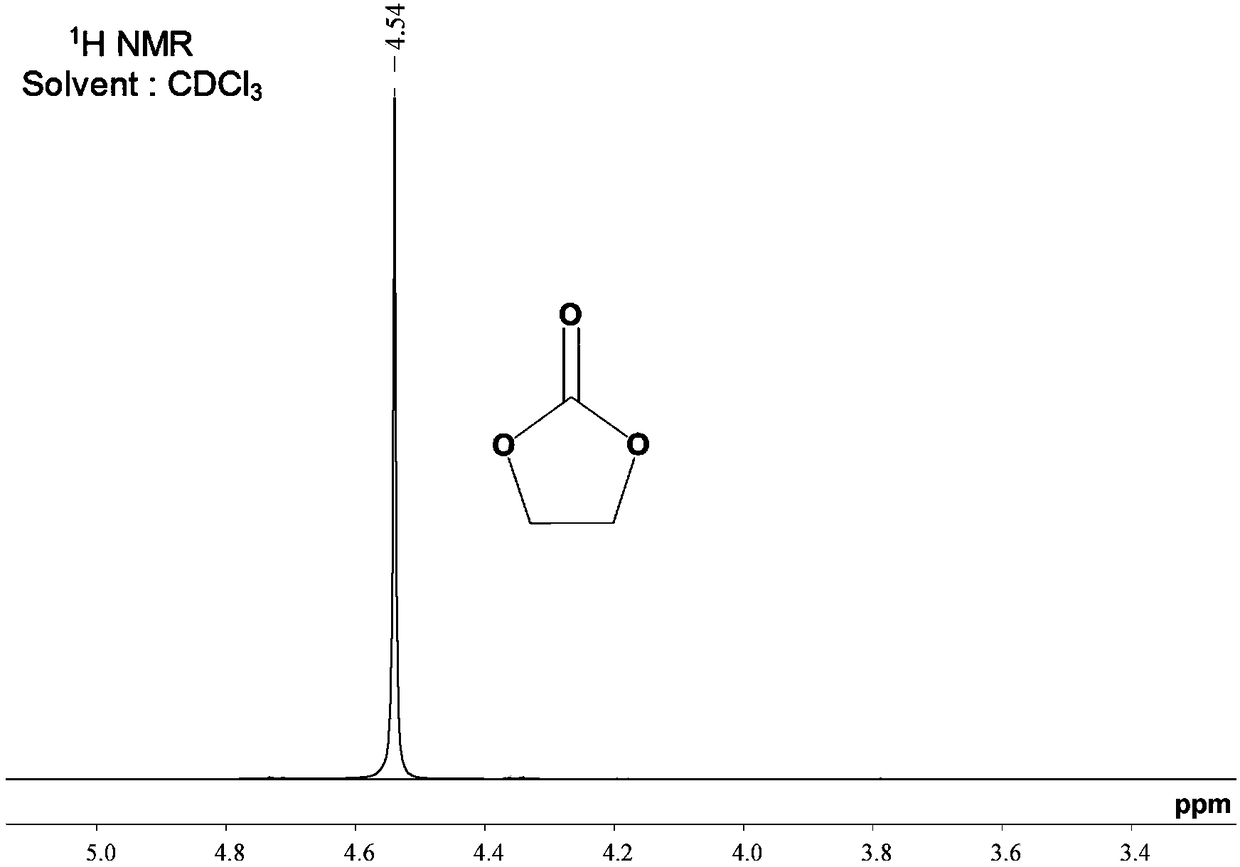
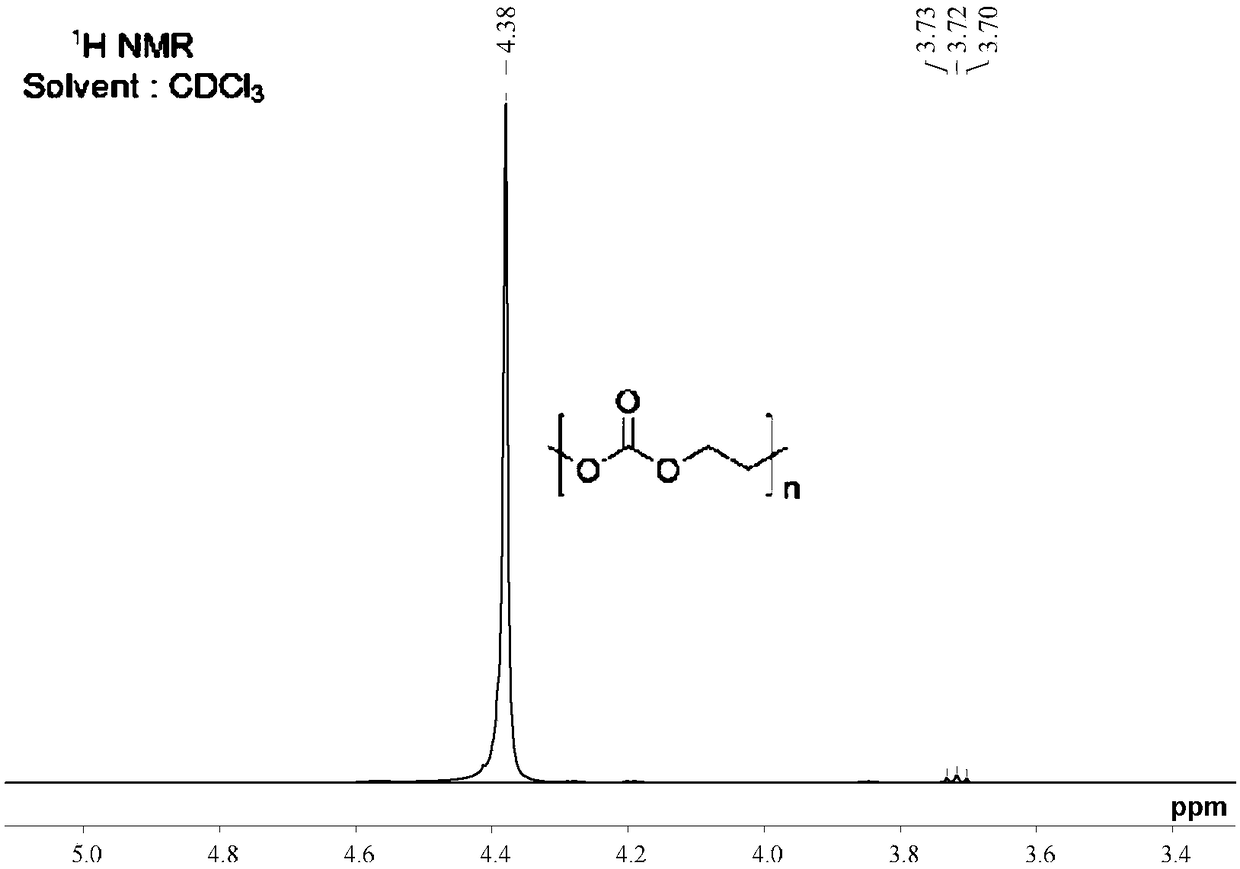
[0064] Embodiment 1: the preparation method of polyvinyl carbonate
[0065] The reaction formula is as follows:
[0066]
[0067] Add triphosgene (0.1mol) and 200mL o-dichlorobenzene into a three-neck flask equipped with a mechanical stirrer, a condenser, and a constant pressure dropping funnel, and stir to dissolve the triphosgene. Add ethylene glycol (0.40 mol), dipotassium hydrogen phosphate (0.60 mol), and triethylamine (catalytic amount) into the bottle, and reflux for 16 hours after the addition is complete. Remove insoluble impurities by filtration, extract impurities and by-products dissolved in the solvent with water, and concentrate under reduced pressure to remove the solvent to obtain an intermediate product.
[0068] Add the intermediate product and 200 mL o-dichlorobenzene into the three-necked flask, and stir to dissolve it. Ethyl chloroformate (0.1mol) was added to the bottle, and the reaction was refluxed for 2h to stop the reaction. Remove insoluble impur...
Embodiment 2
[0071] Add ethylene glycol (0.40mol), dipotassium hydrogen phosphate (0.60mol), and triethylamine (catalytic amount) in the there-necked flask equipped with mechanical stirring, condenser, and constant pressure dropping funnel, and start stirring to make it well mixed. Then add triphosgene (0.1 mol) o-dichlorobenzene solution into the bottle through a constant pressure dropping funnel, and reflux for 16 hours after the addition is complete. The insoluble impurities were removed by filtration, the impurities dissolved in the solvent were extracted with water, and the solvent was removed by concentration under reduced pressure to obtain an intermediate product.
[0072] Add the intermediate product and 200 mL o-dichlorobenzene into the three-necked flask, and stir to dissolve it. Ethyl chloroformate (0.1mol) was added to the bottle, and the reaction was refluxed for 2h to stop the reaction. Remove insoluble impurities by filtration, wash with water three times, and collect the...
Embodiment 3
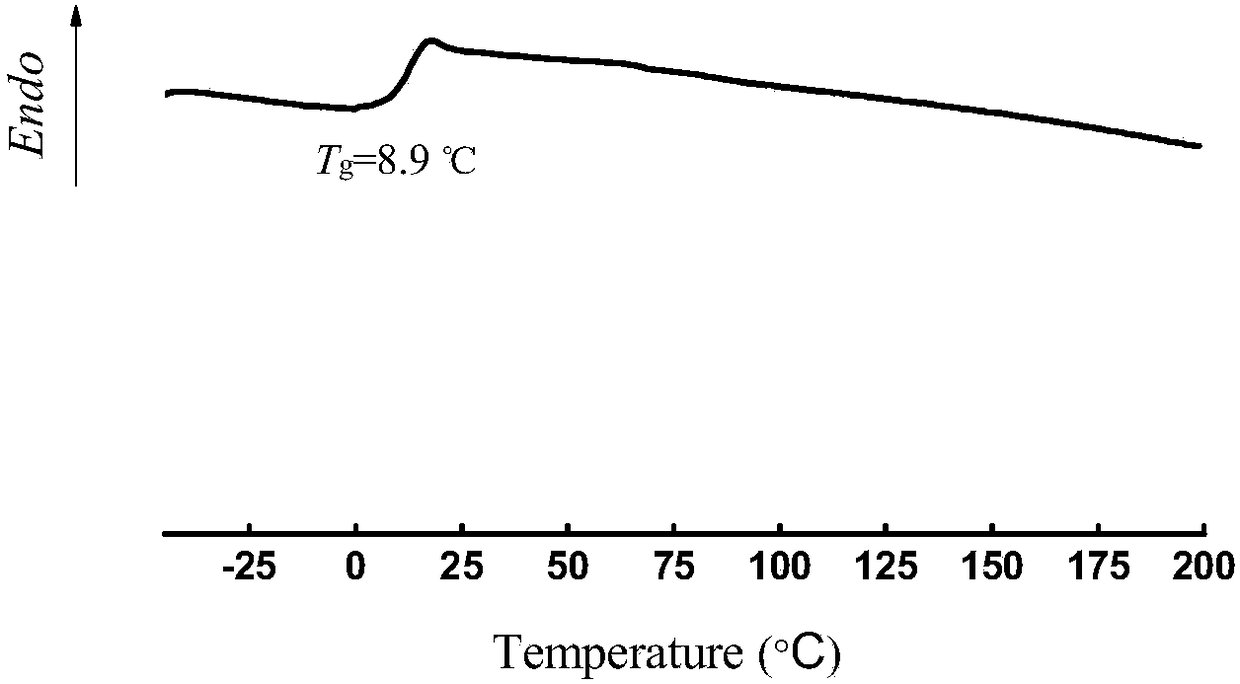
[0076] The product obtained in embodiment 3-8 is polyvinyl carbonate, and reaction device used is identical with embodiment 1, other experimental conditions (solvent, catalyzer, acid-binding agent, end-capping agent, feeding amount, reaction time and temperature of reaction) and The product yields are shown in Table 1 below.
[0077] Table 1. Synthesis results of polyvinyl carbonate:
[0078]
[0079]
[0080] The results of Examples 3-8 show that the molecular weight of the product can be changed by changing the time of the polycondensation reaction, the reaction temperature, the type of catalyst and the type of acid-binding agent, so that the molecular weight of the product can be controlled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com