High performance liquid chromatograph method used for measuring content of netilmicin sulfate
A technology of netilmicin sulfate and high performance liquid chromatography, which is applied in the direction of measuring devices, analysis materials, material separation, etc., can solve the problems of damage to instruments and chromatographic columns, large consumption, and impact on service life, and achieve a wide linear range , high recovery and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 mobile phase and solvent investigation
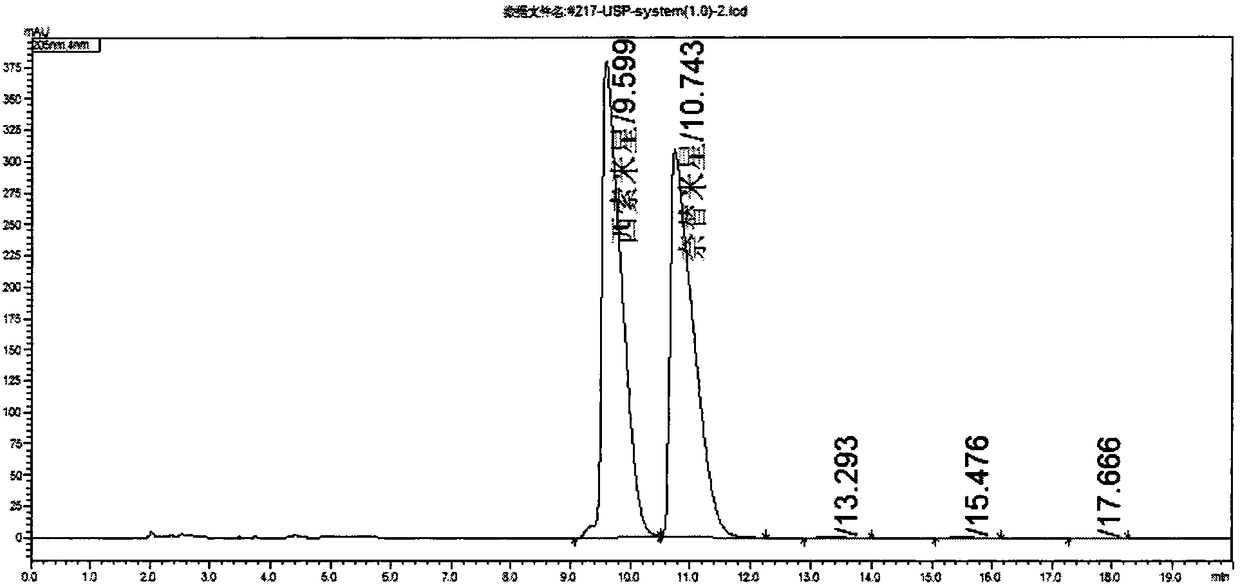
[0045] System suitability solution: get two parts of netilmicin standard substance and sisomicin standard substance each about 17mg, put in 10ml volumetric flask, USP40 method adopts mobile phase to dissolve and dilute to scale, the method of the present invention adopts water to dissolve and Dilute to the mark.
[0046] The mobile phase composition under the USP40 content item is sodium heptanesulfonate dilute phosphoric acid solution (20.22g / L sodium heptanesulfonate is dissolved in 0.5% dilute phosphoric acid solution): acetonitrile=62:38.
[0047] The mobile phase of the method of the present invention consists of sodium heptanesulfonate buffer (6g / L sodium heptanesulfonate+13.6g / L potassium dihydrogen phosphate, phosphoric acid to adjust the pH to 2.5): acetonitrile=77:23.
[0048] USP method and the system suitability solution typical chromatogram of the gained method of the present invention are as figure 1 ...
Embodiment 2
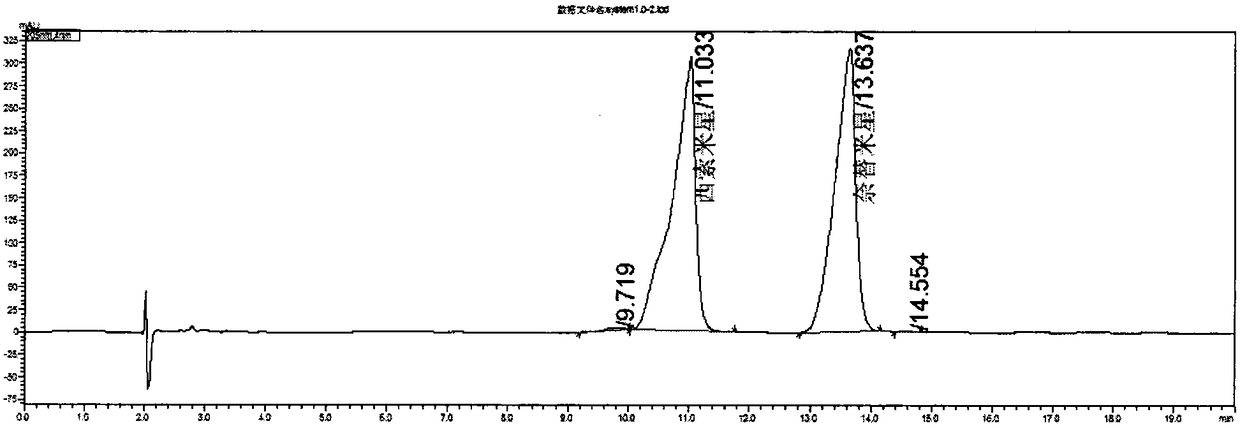
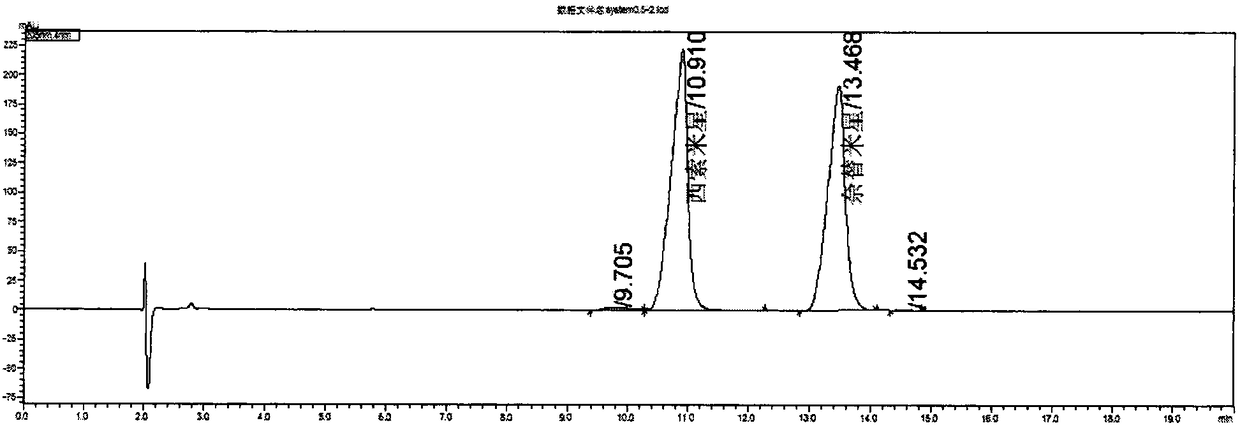
[0052] Embodiment 2 system suitability solution concentration investigation ( image 3 with 4 )
[0053] Prepare system suitability solutions with concentrations of 0.5mg / ml and 0.25mg / ml, inject samples and record chromatograms respectively, and the chromatographic parameters of system suitability solutions with different concentrations are shown in Table 2:
[0054] Table 2 Chromatographic parameters of system suitability solutions at different concentrations
[0055]
[0056] According to the three parameters of resolution, number of theoretical plates and tailing factor in Table 2, the system suitability concentration was determined to be 0.25 mg / ml.
Embodiment 3
[0057] Embodiment 3 specificity investigation ( Figure 5 with Image 6 )
[0058] A reference substance solution with a concentration of 0.25 mg / ml was prepared, and the reference substance solution and water were injected into samples respectively and the chromatograms were recorded. The results showed that the method of the present invention had strong specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com