fe 3+ Molecular fluorescence test agent and preparation method thereof
A testing agent, fluorescence technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as small Stokes shift, achieve high quantum yield, good test accuracy and sensitivity, good The effect of light stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] According to another aspect of the present invention, there is also provided a Fe 3+ The preparation method of molecular fluorescence testing agent, it comprises the following steps:
[0039] In step S1, compound A and compound B are condensed to form compound C; the structures of compound A, compound B and compound C are as follows, wherein X in compound A is a halogen:
[0040]
[0041] Step S2, subjecting compound C to a hydrolysis reaction to obtain Fe 3+ Molecular fluorescence test reagent:
[0042]
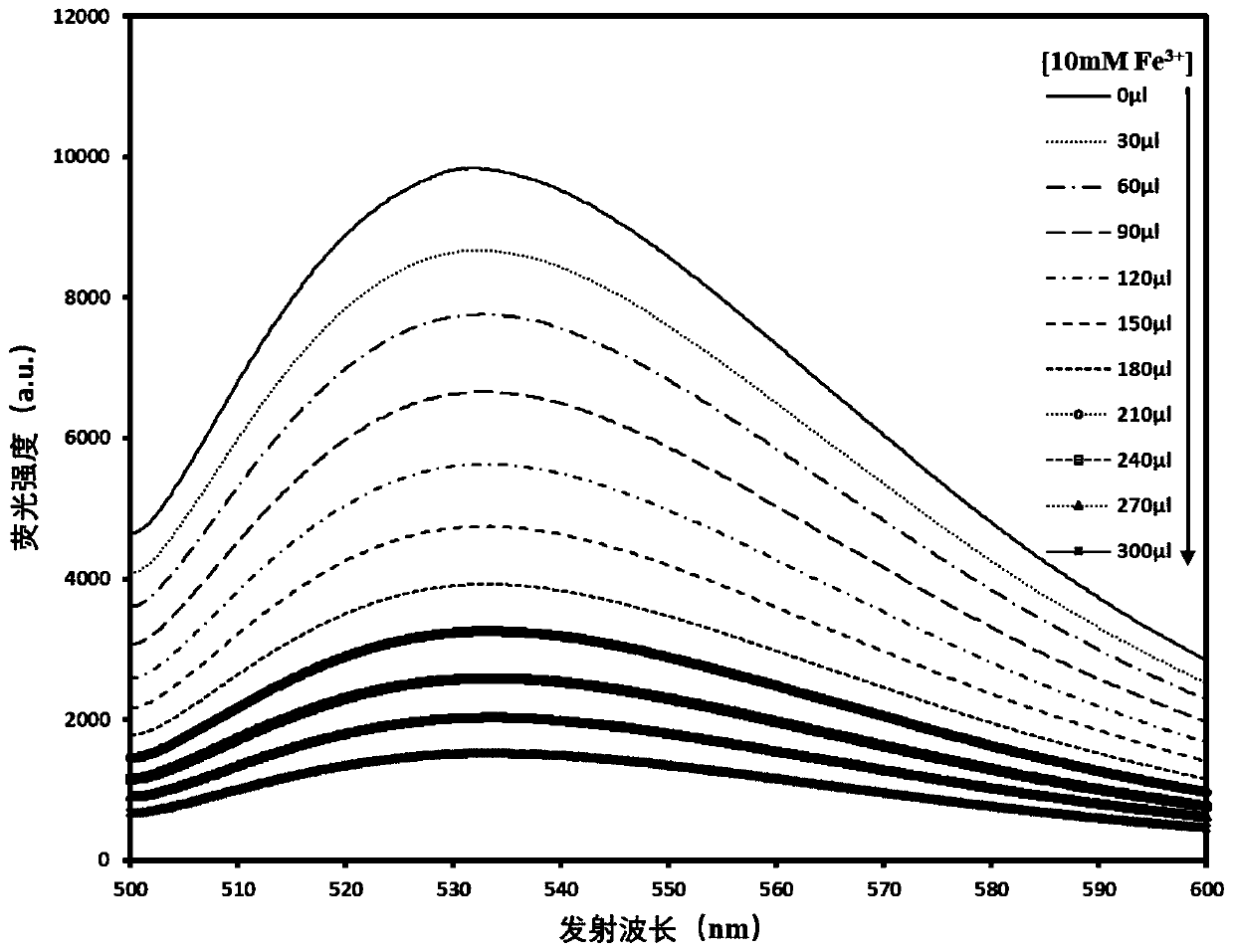
[0043] As mentioned above, the Fe prepared by the modified preparation method of the present invention 3+ Molecular fluorescent test reagents have both better test accuracy and sensitivity. And in this preparation method, compound C is prepared by condensation reaction of compound A and compound B first, and then prepared by hydrolysis of compound C to obtain Fe 3+ Molecular fluorescence testing reagent, the reaction route is simple, the cost is low, and the...
Embodiment 1
[0068] Synthesis of compound 1
[0069]
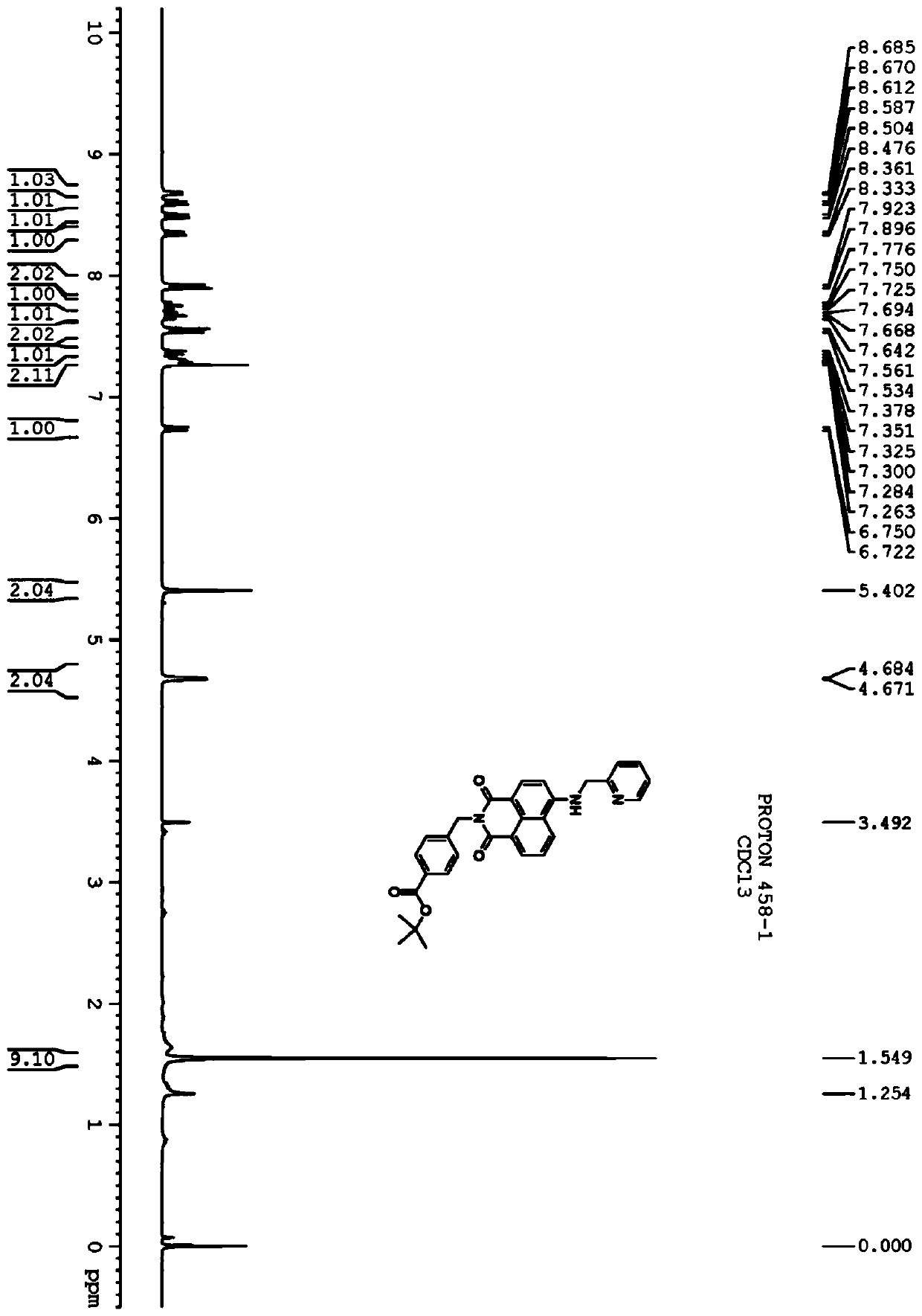
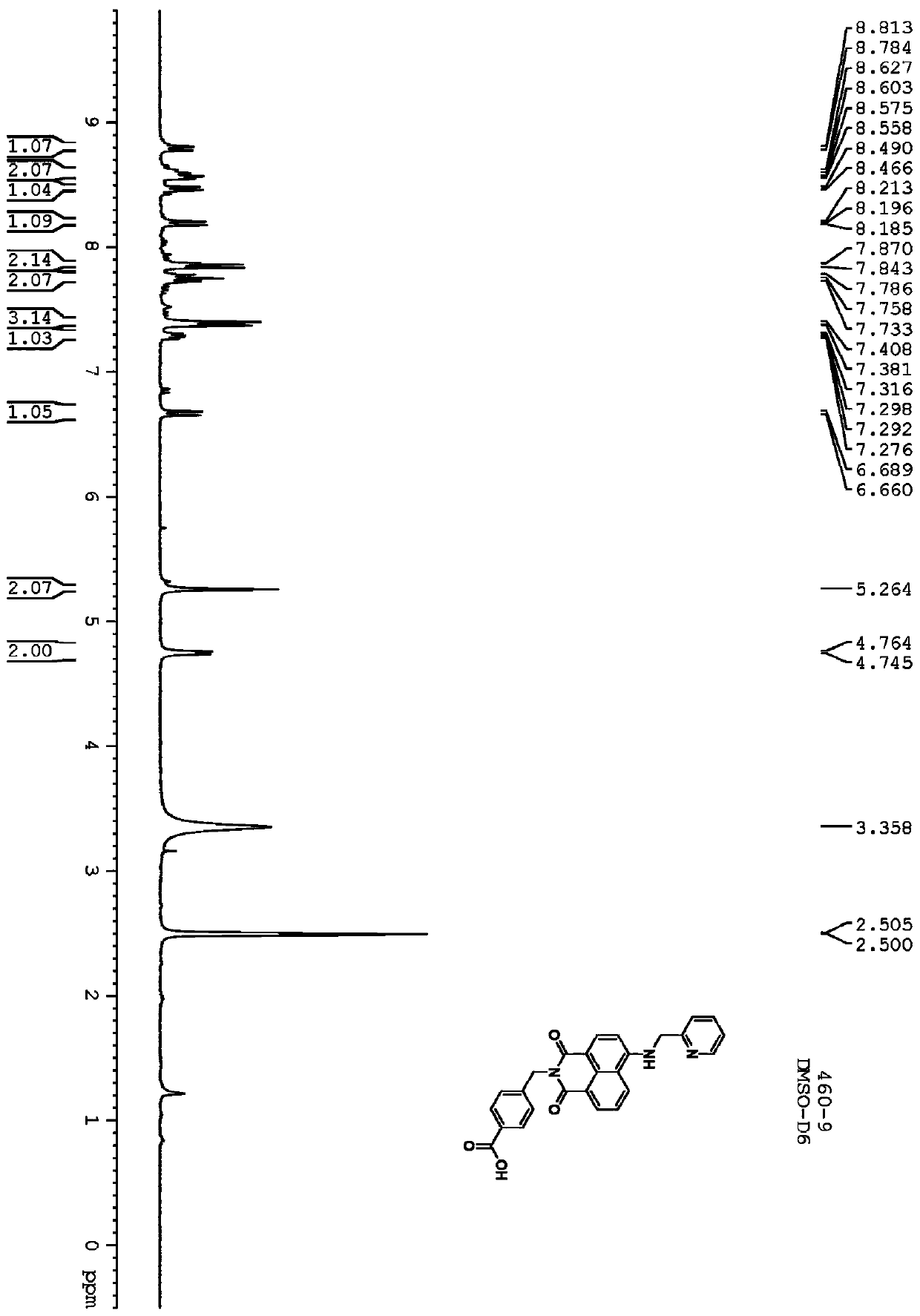
[0070] 4-cyanobenzoic acid (80 g, 544 mmol) was dissolved in anhydrous CH 2 Cl 2 (1000mL). Add oxalyl chloride (104 mL, 816 mmol) and 10 mL of dimethylformamide (DMF). The resulting reaction mixture was stirred at room temperature for 1 hour until gas evolution ceased. Solvent was removed. The resulting residue was treated with 600 mL of a pyridine / tert-butanol mixture (1:1) and stirred at room temperature for 6 hours. The solvent was evaporated under reduced pressure, and the green residue was suspended in H 2 O middle. The aqueous suspension was extracted with ethyl acetate (3 x 500 mL). The combined organic layers were washed with 10% KHSO 4 (2×500mL), H 2 O(500mL)NaHCO 3 (500mL), H 2 O (500 mL) and brine (500 mL) washed. Solvent with Na 2 SO 4 Dry and evaporate. The crude product was purified by column chromatography using ethyl acetate / petroleum ether (1:4) to give white solid 58g (yield 52%).
[0071] Synthesis...
Embodiment 2
[0091] The technique of each step in this embodiment is the same as Example 1, and the only difference is: in the synthetic process of compound 1, adjust the consumption of oxalyl chloride, make the mol ratio of 4-cyanobenzoic acid and oxalyl chloride be 2:3, The volume ratio between pyridine and tert-butanol is 1.1:1. The yield of the final compound 1 was 49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com