Method for preparing 2-nitryl-4-methylsulphonylbenzoic acid
A technology of methylsulfonylbenzoic acid and methylsulfonyltoluene is applied in the field of preparation of 2-nitro-4-methylsulfonylbenzoic acid, and can solve the problems of difficulty in losing electrons, low product yield, low HOMO energy and the like, To achieve the effect of easier reaction and less difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
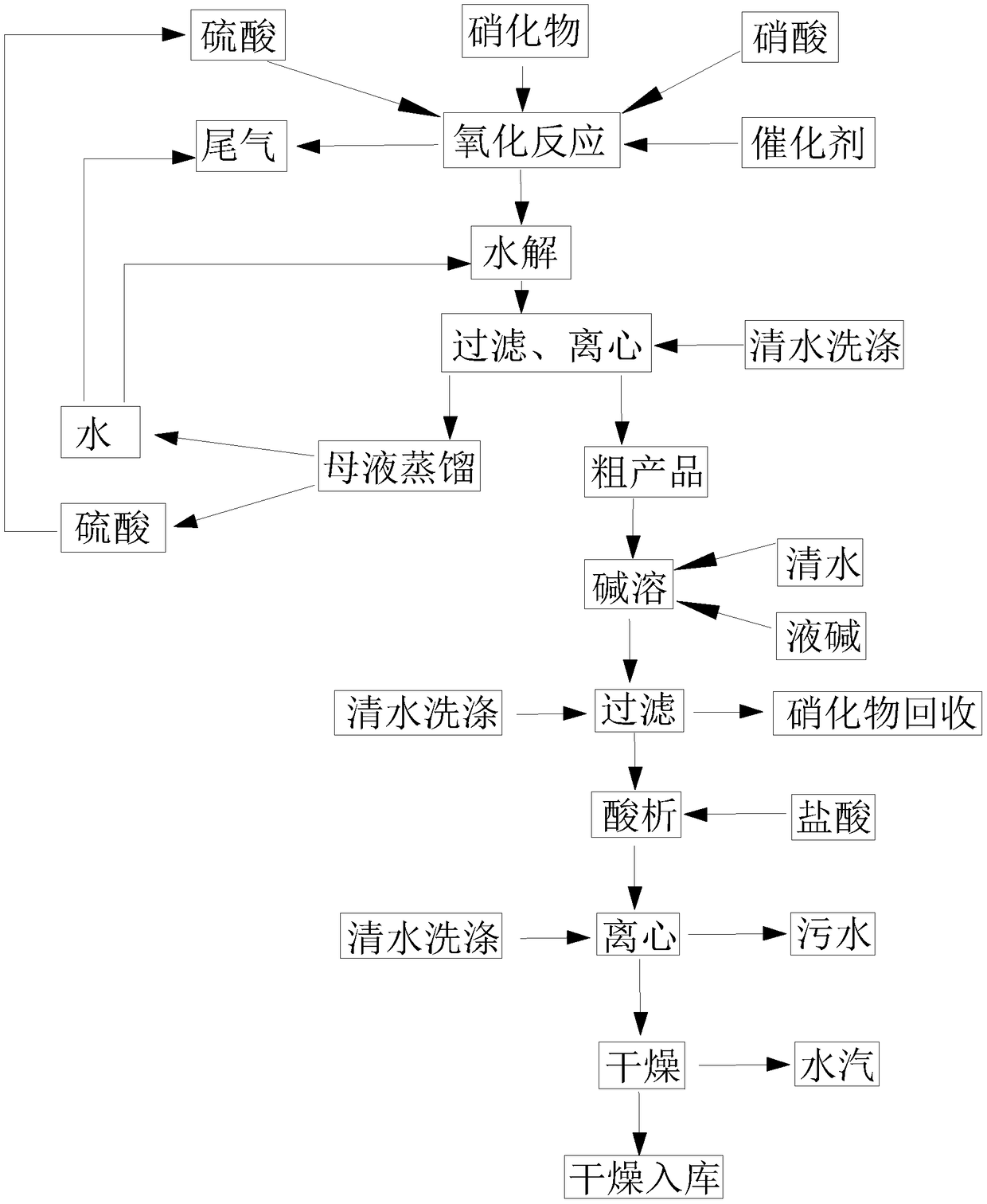
[0034] S1: Mix 650kg of nitrate 2-nitro-4-thiamphenicol toluene with 2000kg of 70% sulfuric acid and 430kg of 65% nitric acid, stir evenly, and add 2kg of catalyst vanadium pentoxide to it, stir, and carry out oxidation reaction ; The tail gas nitric oxide generated during the reaction process is absorbed by water.
[0035] S2: Add 800kg of water to the mixed liquid prepared in S1, stir, and carry out hydrolysis reaction.
[0036] S3: Add 300kg of water to the mixture prepared in S2, stir, filter, and centrifuge to obtain about 600kg of crude product and 3447kg of mother liquor. The mother liquor is distilled to obtain water and sulfuric acid respectively, and the recovered water can be used for the hydrolysis reaction in S2, and can also be used to absorb the tail gas in S1; the recovered sulfuric acid can be used as the reactant sulfuric acid in S1.
[0037] S4: Add 220 kg of liquid caustic soda (40% sodium hydroxide) and 1500 kg of clear water to the crude product obtained...
Embodiment 2
[0043] S1: Mix 650kg of nitrate 2-nitro-4-thiamphenicol toluene with 1500kg of 70% sulfuric acid and 400kg of 65% nitric acid, stir evenly, and add 2kg of catalyst vanadium pentoxide to it, stir, and carry out oxidation reaction ; The tail gas nitric oxide generated during the reaction process is absorbed by water.
[0044] S2: Add 1000kg of water to the mixed liquid prepared in S1, stir, and carry out hydrolysis reaction.
[0045] S3: Add 300kg of water to the mixture prepared in S2, stir, filter, and centrifuge to obtain about 550kg of crude product and 3300kg of mother liquor. The mother liquor is distilled to obtain water and sulfuric acid respectively, and the recovered water can be used not only for the hydrolysis reaction in S2, but also for absorbing the tail gas in S1; the recovered sulfuric acid can be used as reactant sulfuric acid in S1.
[0046] S4: Add 220 kg of liquid caustic soda (45% sodium hydroxide) and 1500 kg of clear water to the crude product obtained i...
Embodiment 3
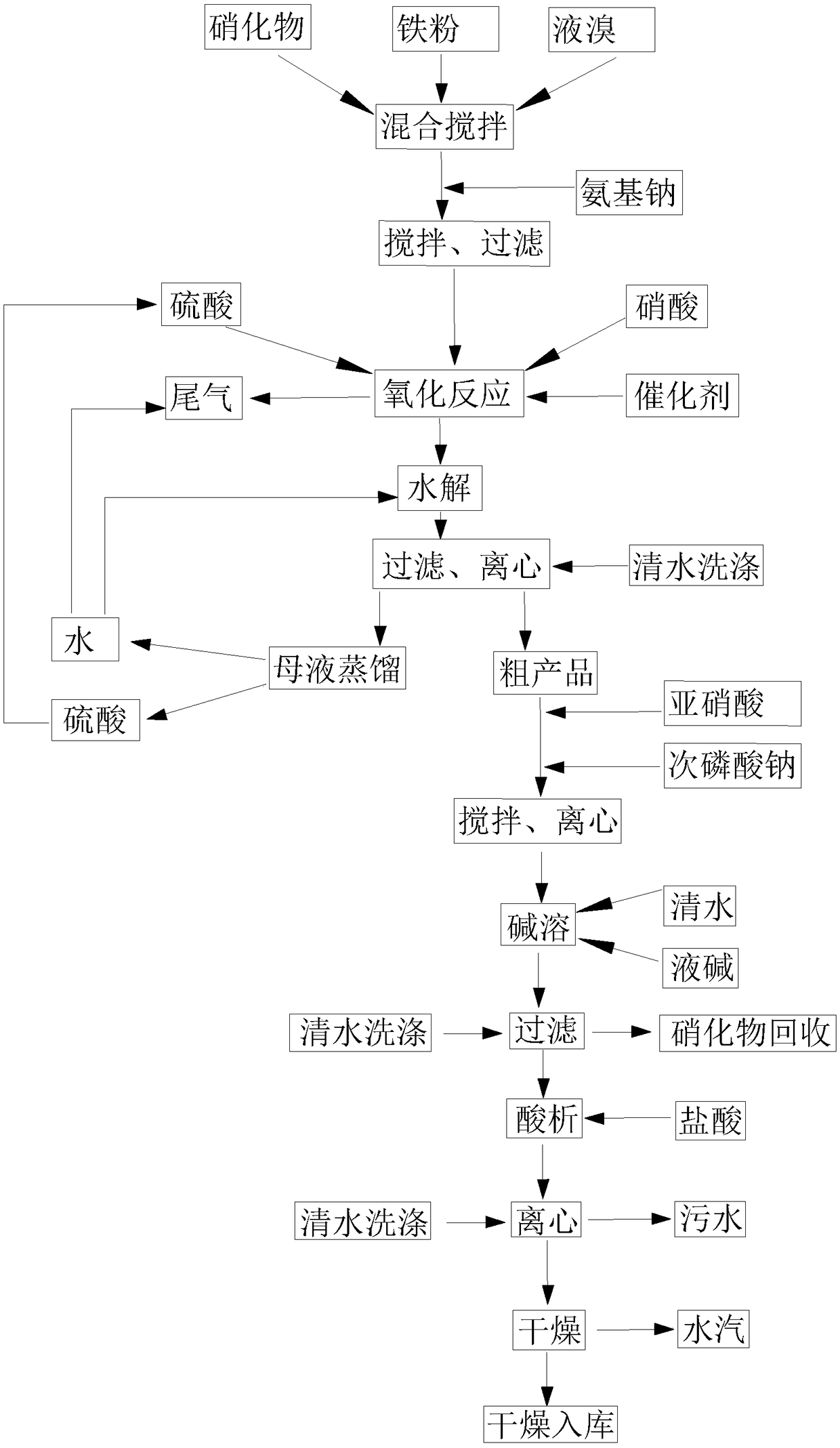
[0052] S1': Mix 650 kg of nitrate 2-nitro-4-thiamphenicol toluene with 10 kg of iron powder, mix and stir, and gradually add 300 kg of liquid bromine to it, stir and mix evenly, after the reaction, add 280 kg of sodium amide to it, The reaction was sufficient, and liquid products and iron powder precipitation remained in the container.
[0053] S1: Mix the liquid product in S1 with 2000kg of 70% sulfuric acid and 430kg of 65% nitric acid, stir evenly, and add 0.5kg catalyst vanadium pentoxide to it, stir, and carry out oxidation reaction; Nitrogen oxides are absorbed with water.
[0054] S2: Add 800kg of water to the mixed liquid prepared in S1, stir, and carry out hydrolysis reaction.
[0055] S3: Add 300kg of water to the mixture prepared in S2, stir, filter, and centrifuge to obtain about 850kg of crude product and 3300kg of mother liquor. The mother liquor is distilled to obtain water and sulfuric acid respectively, and the recovered water can be used for the hydrolysis re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com