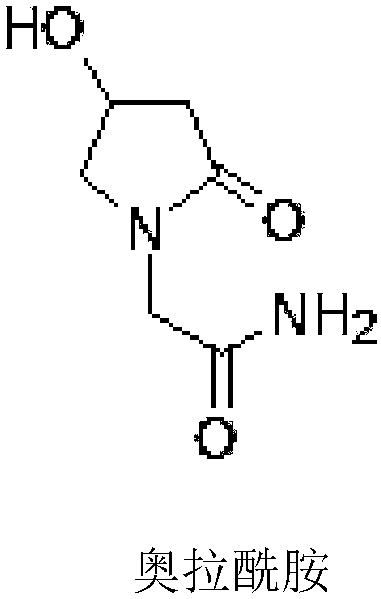Oxiracetam oral disintegrating preparation and preparation method thereof
An olamide and oral disintegration technology, used in pill delivery, pharmaceutical formulations, cardiovascular system diseases, etc., to achieve the effects of increased absorption, moderate hardness and good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Get 17 parts of absolute ethanol, under stirring state, add 1 part of Olamide chemical bulk drug (provided by Chongqing Fuan Pharmaceutical Co., Ltd., purity 99.5%) and 5 parts of hydroxypropyl methylcellulose phthalate (provided by Guangzhou Shuoheng Biological Technology Co., Ltd.), stirred until dissolved, and then spray-dried in a spray dryer (Huawei Technology Co., Ltd.). ~81°C; grind the dried olaamide solid dispersion to obtain the product.
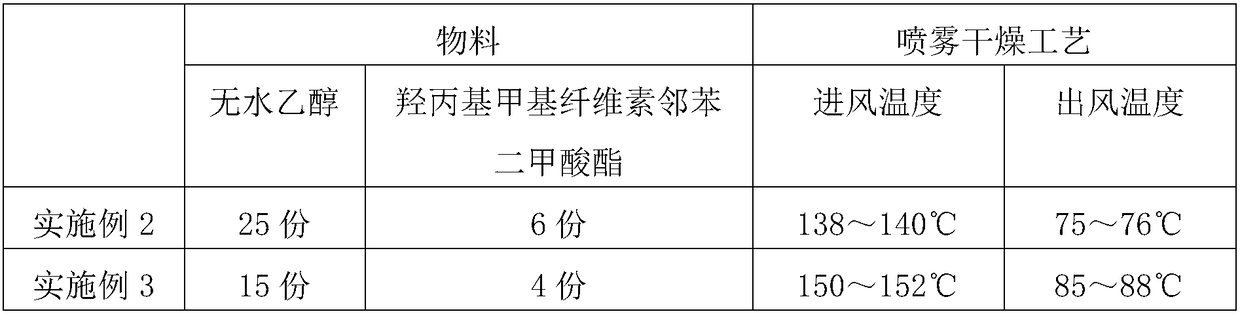
[0032] Referring to the preparation method of Example 1, run Examples 2-5 according to the parameters in Table 1 below to prepare the solid dispersion of alamide. All the raw materials of Olamide used are 1 part.
[0033] Table 1 Preparation of Olamide Solid Dispersion
[0034]
[0035]
Embodiment 6
[0037] The release of the orally disintegrating preparation containing olaamide solid dispersion in gastric juice and intestinal juice of the present invention was investigated.
[0038] ①Simulated gastric juice
[0039] Measure 900ml of 0.1mol / L hydrochloric acid solution, pour it into the dissolution cup, heat to keep the solution temperature at 37±0.5°C, quantitatively and accurately weigh 6 parts of the solid dispersion samples of alamide in Examples 1-5, and put them into the basket , start the machine to run at a speed of 100rpm, take samples at 10min, 30min, 60min, 90min, and 120min respectively, and filter through a 0.8um microporous water-phase filter membrane, from sampling to filtration is completed within 30s, and the sample is measured at a wavelength of 276nm Absorbance, and using the corresponding solvent as a blank control, the dissolution percentage of olaamide was calculated by the external standard method. The drug release of olaamide solid dispersion in si...
Embodiment 7
[0049] Prescription: 59g of the solid dispersion of alaramide prepared in Example 1, 10g of pregelatinized starch, 12g of sodium carboxymethyl starch, 8g of low-substituted hydroxypropyl cellulose, 8g of polyvinylpyrrolidone, 1g of xylitol, and 2g of micropowdered silica gel .
[0050] Preparation:
[0051] (1) Material sieving: pass the solid dispersion of oracamide, pregelatinized starch, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone, xylitol, and micropowdered silica gel through a 100-mesh sieve respectively, spare;
[0052] (2) Mixing and granulation: Add the sieved solid dispersion of oramide, filler, and disintegrating agent to the wet granulator and mix evenly, start the stirring paddle to mix for 5 minutes; (20% polyvinylpyrrolidone ethanol solution made of ethanol), start the stirring paddle, mix the granulating paddle to prepare soft material for 7 minutes, and discharge when the particle size is about 80 mesh; wherein, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com