A stable lead-free low bandgap all-inorganic perovskite a 2 pdx 6 Nanocrystal and its preparation method
A nanocrystal, low bandgap technology, applied in the field of preparation of new nanomaterials, can solve the problems of narrow visible light absorption range, narrow size distribution, poor stability, etc., and achieve the effects of good dispersion, uniform size and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
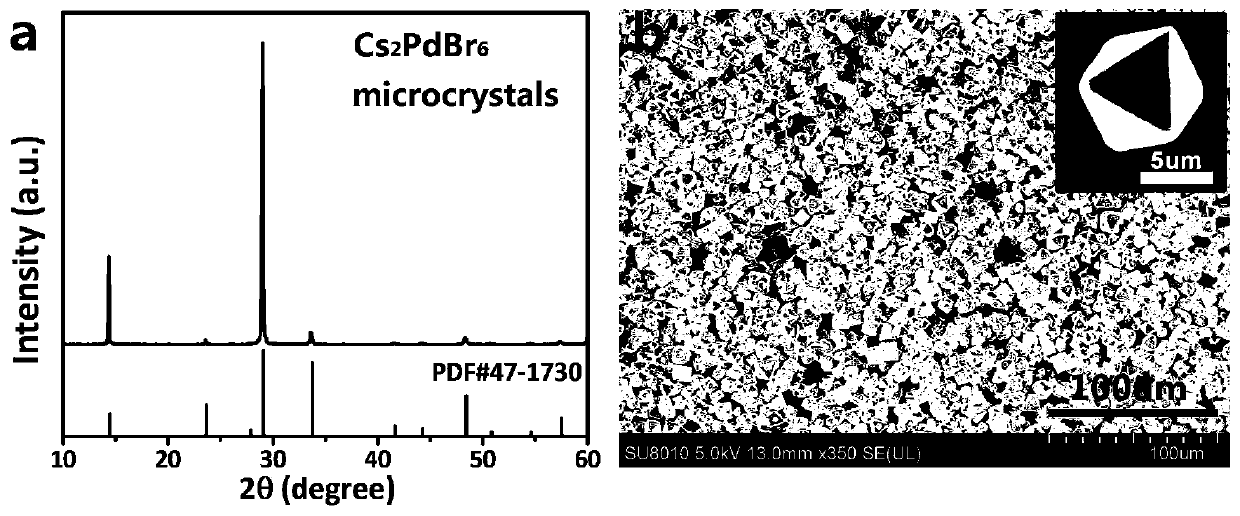
[0039] A stable lead-free low bandgap all-inorganic perovskite Cs 2 PdBr 6 The preparation method of microcrystal, comprises the following steps:
[0040] Step 1, 0.851g CsBr, 0.532g PdBr 2 , 10mL HBr was added into the three-necked flask at the same time and mixed, then the system was heated and stirred at 85°C for 5min to ensure that the PdBr 2 and CsBr were all dissolved to obtain a clear and transparent solution;
[0041] Step 2, prepare 20mL DMSO-HBr mixed solution as an oxidant, wherein DMSO accounts for 10% of the total volume;
[0042] Step 3, warm up the clear solution in step 1 to 120 °C, then quickly add 20 mL of DMSO-HBr mixed solution, immediately generate a large amount of Cs 2 PdBr 6 Microcrystalline, after 30s of reaction, filter the product while it is hot, wash it with deionized water while filtering, and finally bake the product in an oven at 70°C for 30 minutes to obtain high-purity Cs 2 PdBr 6 Microcrystalline.
[0043] like figure 1 As shown in a...
Embodiment 2
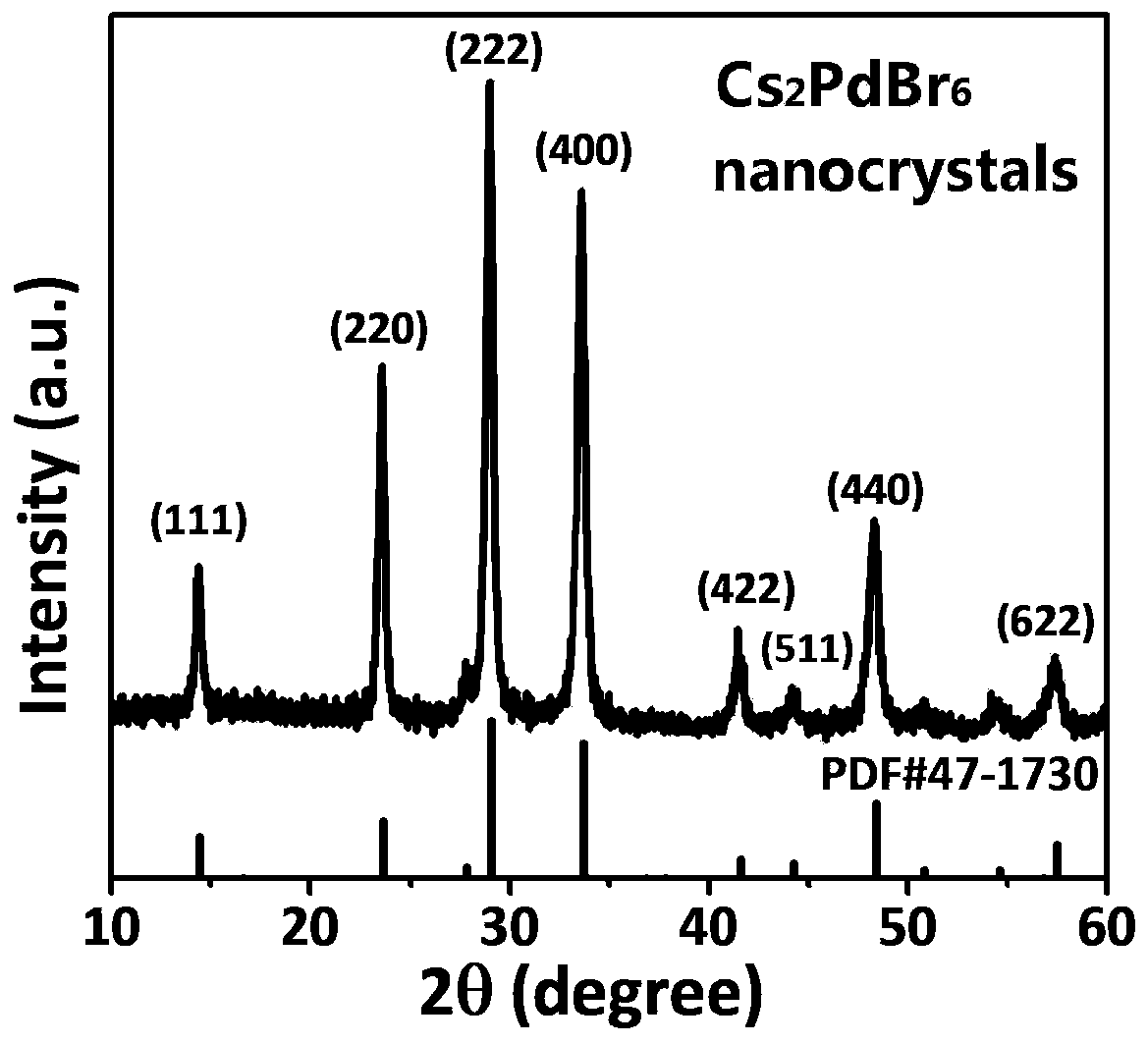
[0045] Using the Cs prepared in Example 1 2 PdBr 6 Microcrystals are used as raw materials, which are dissolved in DMF to obtain a clear precursor solution, and then an appropriate amount of precursor solution is added to the anti-solvent to prepare Cs 2 PdBr 6 Nanocrystalline. The specific steps are:
[0046] Get the Cs prepared in 10mg embodiment 1 2 PdBr 6 Microcrystals were added to 2mL DMF, and then heated at 130°C for 1min to make Cs 2 PdBr 6 All the microcrystals were dissolved in DMF to obtain a clear precursor solution; finally, 200 μL of the precursor solution was quickly added to 2 mL of the anti-solvent propionic acid that was stirring, and a large amount of black precipitate was generated immediately, and after about 20 seconds, the stirring was stopped. The obtained nanocrystals can be directly used for characterization or testing of various other properties. The size of the obtained nanocrystals is uniform, and no large-scale morphology appears. In addi...
Embodiment 3
[0051] Using the Cs prepared in Example 1 2 PdBr 6 Microcrystals are used as raw materials, which are dissolved in DMF to obtain a clear precursor solution, and then an appropriate amount of precursor solution is added to the anti-solvent to prepare Cs 2 PdBr6 Nanocrystalline. The specific steps are:
[0052] Get the Cs prepared in 10mg embodiment 1 2 PdBr 6 Microcrystals were added to 2mL DMF, and then heated at 130°C to make Cs 2 PdBr 6 All the microcrystals were dissolved in DMF to obtain a clear precursor solution; then 200 μL of the precursor solution was quickly added to 2 mL of the anti-solvent isobutanol that was stirring, and a large amount of black precipitate was generated immediately, and after about 20 seconds, the stirring was stopped.
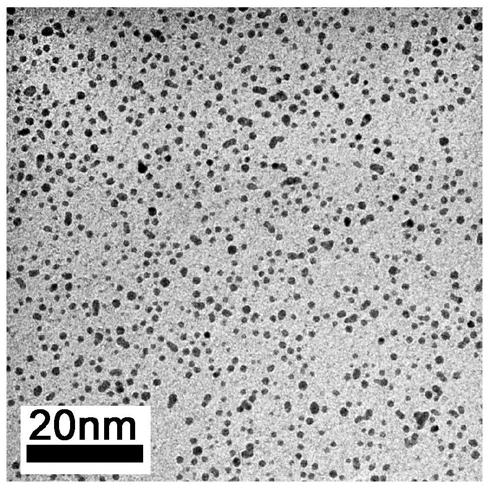
[0053] Figure 7a and Figure 7b Respectively the Cs synthesized in isobutanol in the present embodiment 3 2 PdBr 6 Morphology and size distribution of nanocrystals. It can be seen from the figure that Cs can be prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com