Synthetic method of 1,9-diaminononane
A synthesis method and nonanediamine technology are applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., and can solve problems such as high requirements for production equipment, inability to apply in the field of food, and not yet achieved localization, etc. The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
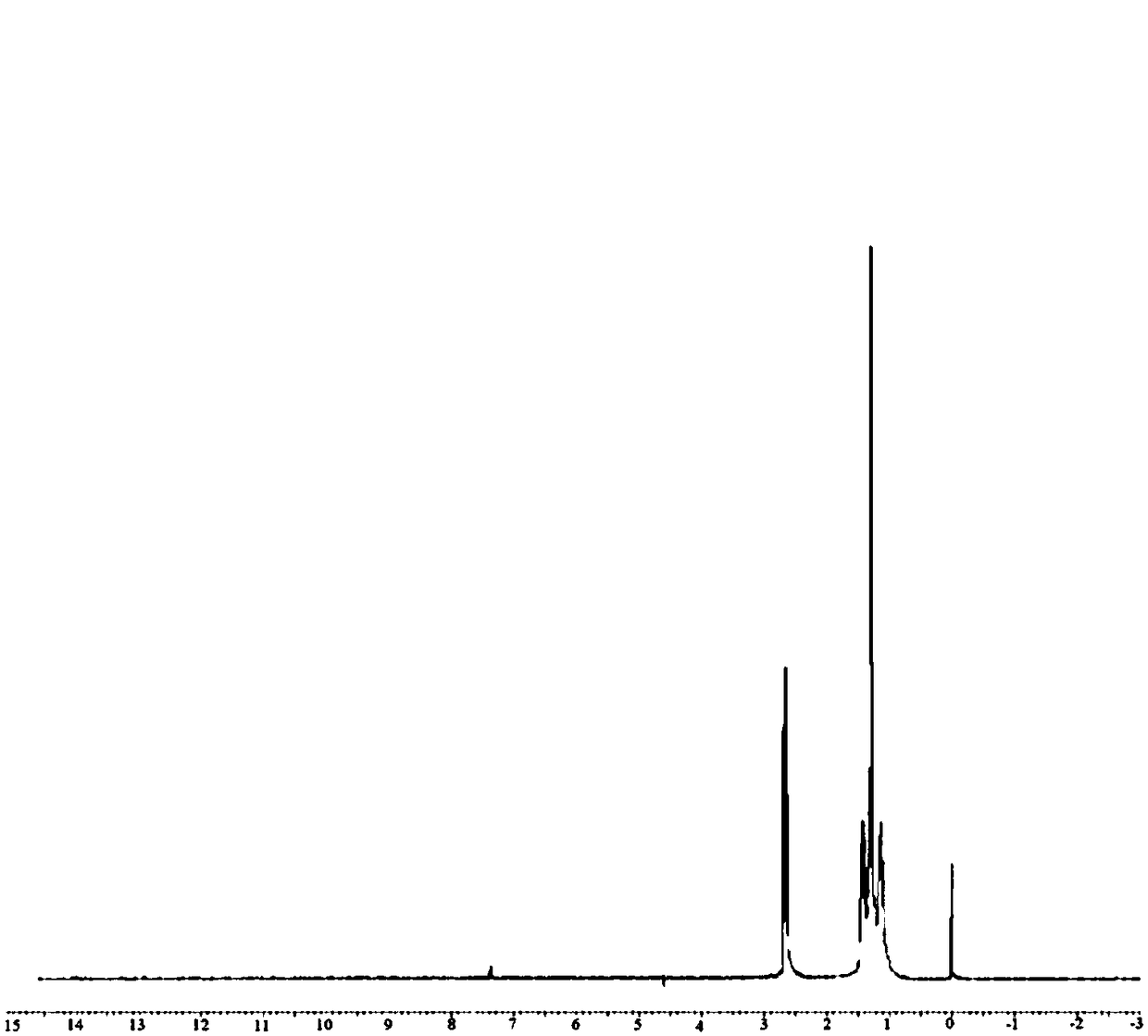
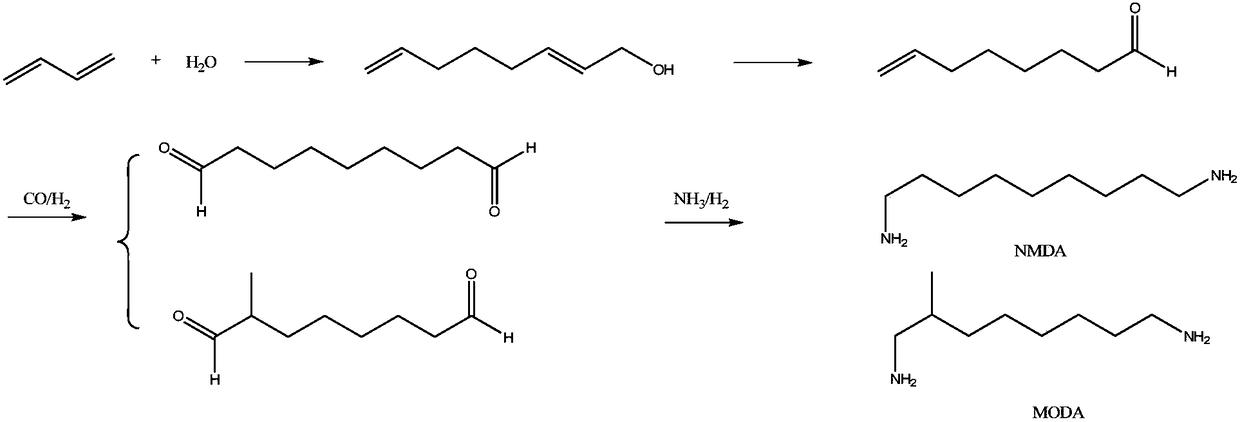
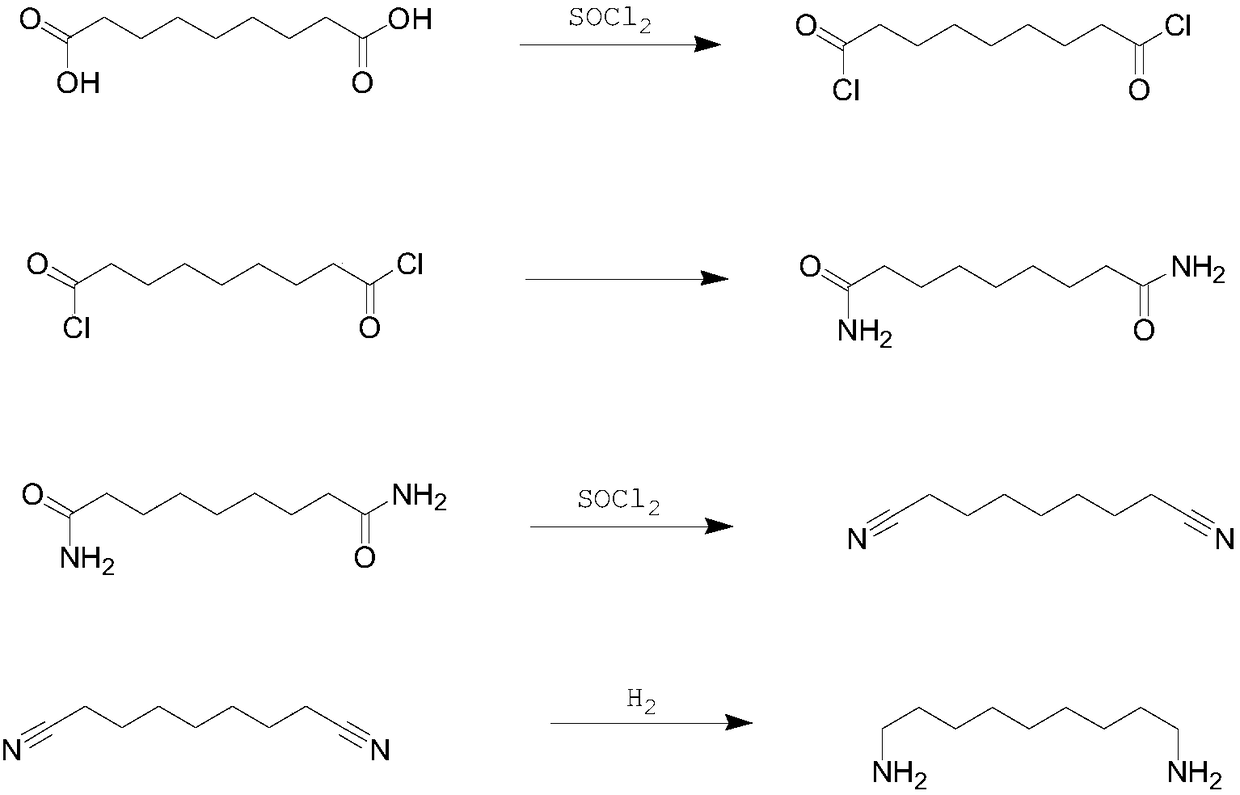
Image
Examples
Embodiment 1
[0057]
[0058] step 1:
[0059] Add 140 ml of methanol and 174 grams of undecanedioic acid into a 500 ml three-necked flask, and slowly add 201 grams of thionyl chloride at room temperature. The temperature was raised to reflux and the reaction was continued for 90 minutes. After the reaction system was naturally cooled to 25°C, the solvent was removed by a rotary evaporator to obtain 192.62 grams of dimethyl undecanedate (GC=99.2%), which was directly used for the next step reaction raw material.
[0060] Step 2:
[0061] Add 192.62 grams of dimethyl undecanedioate, 140 milliliters of methanol, and 12.63 grams of ammonia water (mass fraction=25%) into a 500 ml three-necked flask, and stir and react at 40° C. for 1 hour. The solvent was removed to obtain the intermediate of the second step: 160.5 g of undecanediamide.
[0062] Step 3:
[0063] Add 21.4 grams of undecanediamide to the freshly prepared aqueous solution of sodium hypochlorite (composed of 0.2 mol of sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com