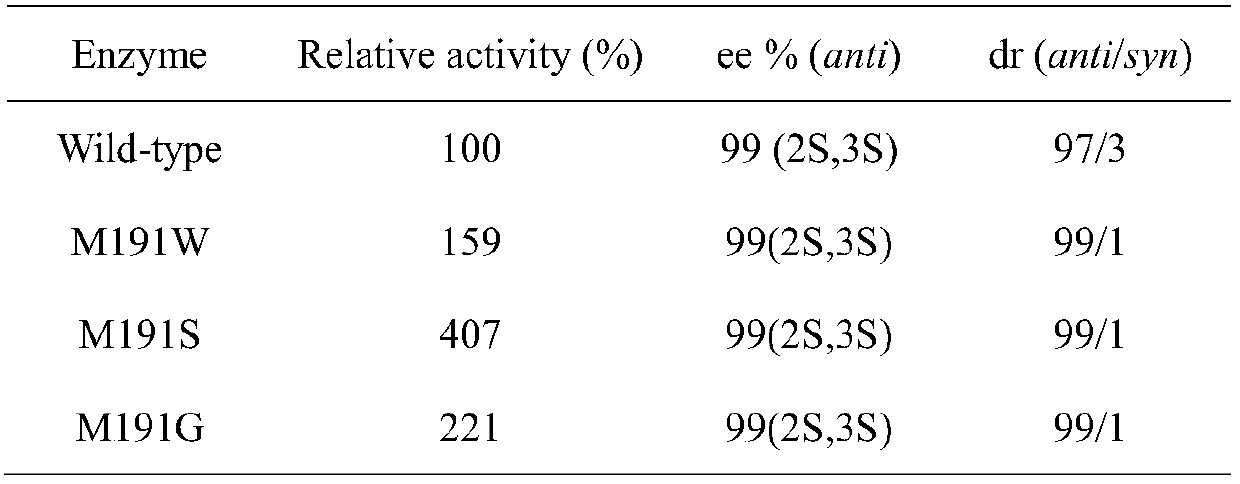
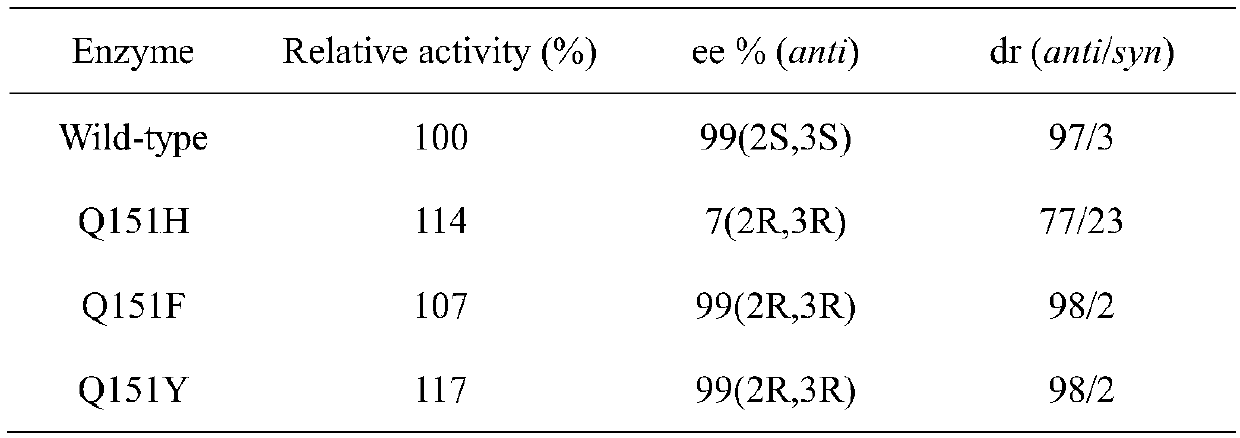
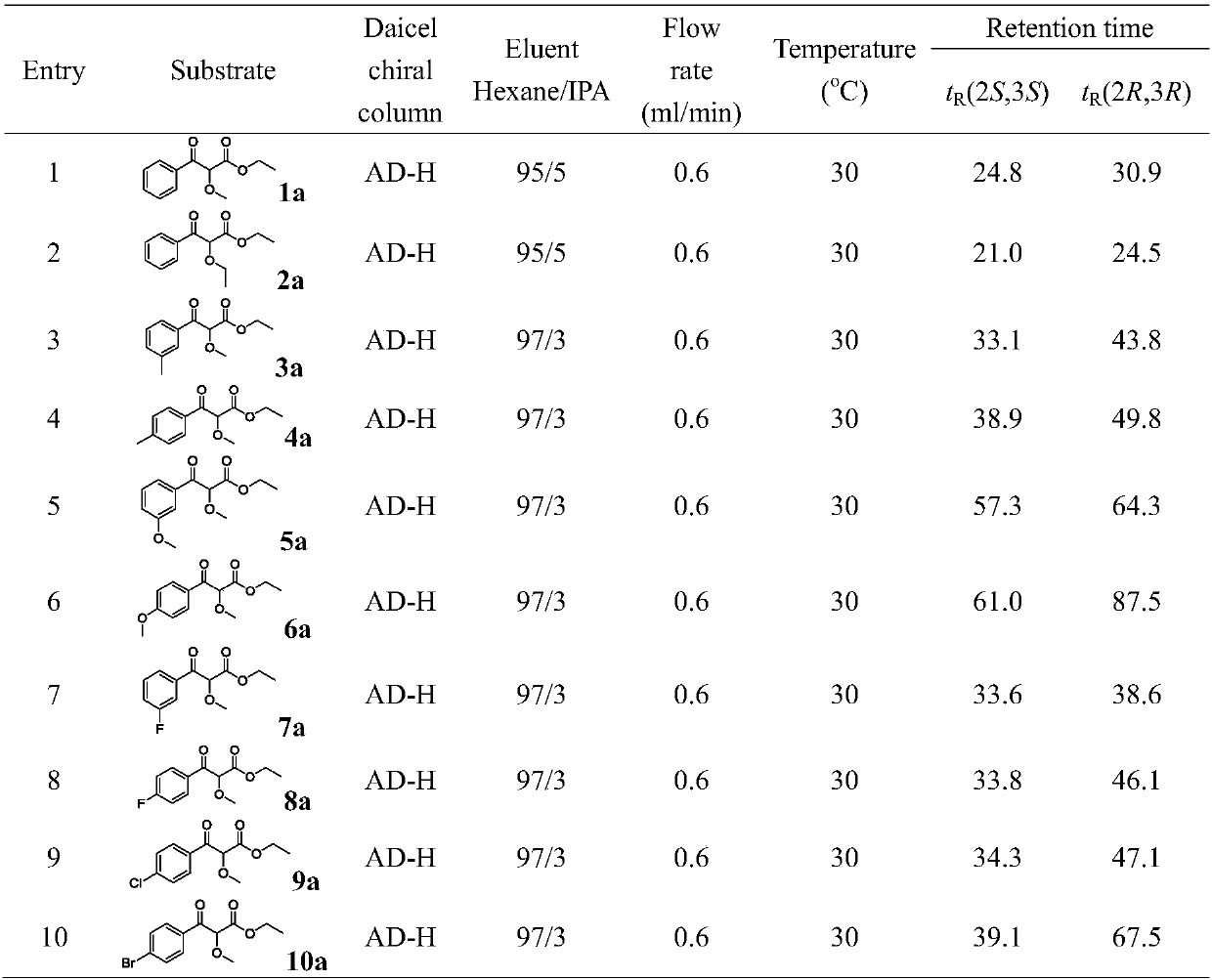
Carbonyl reductase mutant and application thereof
A reductase and mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of inability to meet huge demand, not easy to obtain, and the catalytic activity of enzymes cannot meet industrial production, and achieve the effect of enriching the biocatalysis toolbox
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of a single-point saturation mutation library
[0030] use The site-directed mutagenesis kit performs single-point saturation mutations on the carbonyl reductase ChKRED12 gene (see SEQ ID NO.1).
[0031] The primers used are:
[0032] M191-SM:T7:5′-CCGGGTTTTATCAAAACGGAT NNK ACCCAGGAGTTT-3′
[0033] T7 ter : 5′-AAACTCCTGGGTM NNA TCCGTTTTGATAAAACCCGG-3′
[0034] Q151-SM:T7:5′-ACTGCGGGT NNK ACCAATTATAGCGCAGCGA-3′
[0035] T7 ter : 5′-TCGCTGCGCTATAATTGGT MNN ACCCGCAGT-3′
[0036] The reaction conditions were: pre-denaturation at 98°C for 3 minutes, denaturation at 98°C for 10 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 2 minutes, a total of 25 cycles. After electrophoresis, the gene fragments were recovered with a gel recovery kit. After the recovered fragment was digested with DpnI, it was transformed into Escherichia coli DH10B by electroporation to obtain a cloned mutant library.
Embodiment 2
[0037] Example 2 Screening of Carbonyl Reductase ChKRED12 Mutant Library
[0038]The mutant library clones in Example 1 were collected and the plasmids were extracted, transformed into E. coli expression strain BL21-DE3, spread on LB plates containing kanamycin (50 μg / mL), and cultured for 12 hours. Single clones were picked and placed in a 96-well plate, each well contained 200 μL TB medium (containing 50 μg / mL kanamycin, 0.5 mM IPTG), 37° C., 180 rpm, and cultured with shaking for 18 hours. The 96-well plate replicator replicated each single clone on an LB solid medium plate, cultured at 37°C for 12 hours, and stored in a refrigerator at 4°C. Centrifuge the 96-well plate after bacterial induction and expression at 4°C and 4000 rpm for 10 min, discard the supernatant, and add 200 μL of lysis buffer to each well to resuspend the cells (configuration of lysis buffer: 0.1 M, pH 8.0 potassium phosphate Buffer, 10mg / mL lysozyme, 1μg / mL DNase I, 10mM MgCl 2 ). Place the 96-well ...
Embodiment 3
[0039] The mensuration of embodiment 3 crude enzyme liquid enzyme activity
[0040] 3.1 Preparation of crude enzyme solution
[0041] Pick a single clone into LB (containing kanamycin 50 μg / mL) medium, culture overnight at 37 ° C, transfer to TB (containing kanamycin 50 μg / mL) medium with 1% inoculum size, 37 Cultivate at ℃ for 3h, add 0.5mM IPTG to induce, and continue to culture at 37°C for 18h. The bacteria liquid was centrifuged to collect the bacteria, the cell homogenizer was crushed, and the supernatant was centrifuged to obtain the crude enzyme liquid.
[0042] 3.2 Determination of crude enzyme activity
[0043] Crude enzyme activity assay reaction conditions: 3mg / mL crude enzyme solution (total protein concentration), 1mM NADP + , 0.1 M, pH 8.0 potassium phosphate buffer, 0.1 mL dimethyl sulfoxide, 10 mM substrate ethyl 2-methoxy-3-carbonylphenylpropionate (1a), 5% (w / v) Glucose, 2mg / mL glucose dehydrogenase. React at 40°C and 150rpm for 60min. After the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com