Micro-reaction system and method for continuously preparing (R)-3-hydroxyhex-5-enoate by using same
A hexenoate, micro-reaction technology, applied in sterilization methods, biochemical equipment and methods, sustainable manufacturing/processing, etc., can solve the problem of difficult recovery and reuse of enzyme catalysts, poor stability of free enzymes, and complicated processing operations. and other problems, to achieve the effect of saving the cost of catalyst separation and repeated use, shortening the reaction time, and improving the efficiency of the process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst
[0060] Weigh 5 g of polyvinyl alcohol, 3 g of polyethylene glycol and 35 mL of water into a reaction flask, heat until the solution is clear, cool down to below 50 °C, add 10 mL of crude enzyme solution of carbonyl reductase (15% w / v ) and 5 mL isopropanol dehydrogenase crude enzyme solution (15% w / v), mix well. After mixing, drop the solution onto the polyethylene film with a syringe, and then place it in a blast oven at 35°C to dry for 1 hour to obtain the carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst, which is stored in Store at 4°C for later use.
[0061] In this example, the preparation methods of the crude enzyme solution of carbonyl reductase and crude isopropanol dehydrogenase are incorporated herein for reference by citing Chinese patent application CN107119081A.
Embodiment 2
[0062] Embodiment 2 target product ( R ) Preparation of tert-butyl 3-hydroxy-5-hexenoate
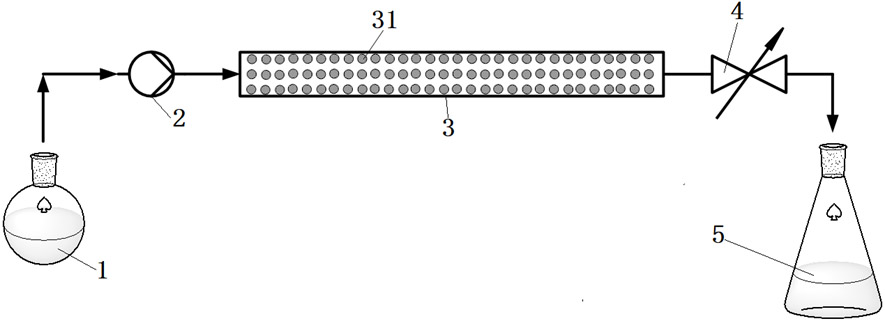
[0063] use as figure 1 In the shown micro-reaction system, the micro-channel reactor 3 is a tubular micro-channel reactor. Weigh 5 g of the carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst prepared in Example 1, and fill it in a tubular microchannel reactor 3 with an inner diameter of 10 mm and a length of 200 mm. Before starting the reaction, pump disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution (pH 7.0) into the packed carbonyl reductase / isopropanol dehydrogenase co-immobilized solution through feed pump 2 at a flow rate of 5 mL / min. In the tubular microchannel reactor 3 of the catalyst 31, rinse for 5 min. Then, the substrate liquid containing 3-carbonyl-5-hexenoic acid tert-butyl ester is pumped into the tubular microchannel reactor 3 from the substrate liquid container 1 by the feed pump 2, and the back pressure of the back pressure ...
Embodiment 3
[0065] Embodiment 3 target product ( R ) Preparation of tert-butyl 3-hydroxy-5-hexenoate
[0066] use as figure 1 In the shown micro-reaction system, the micro-channel reactor 3 is a tubular micro-channel reactor. Weigh 2.5 g of the carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst prepared in Example 1, and fill it in a tubular microchannel reactor 3 with an inner diameter of 5 mm and a length of 200 mm. Before starting the reaction, pump disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution (pH 7.0) into the filled carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst through the feed pump 2 at a flow rate of 5mL / min 31 of the tubular microchannel reactor 3, rinse for 5 min. Then, the substrate liquid containing 3-carbonyl-5-hexenoic acid tert-butyl ester is pumped into the tubular microchannel reactor 3 from the substrate liquid container 1 by the feed pump 2, and the back pressure of the back pressure valve 4 is se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com