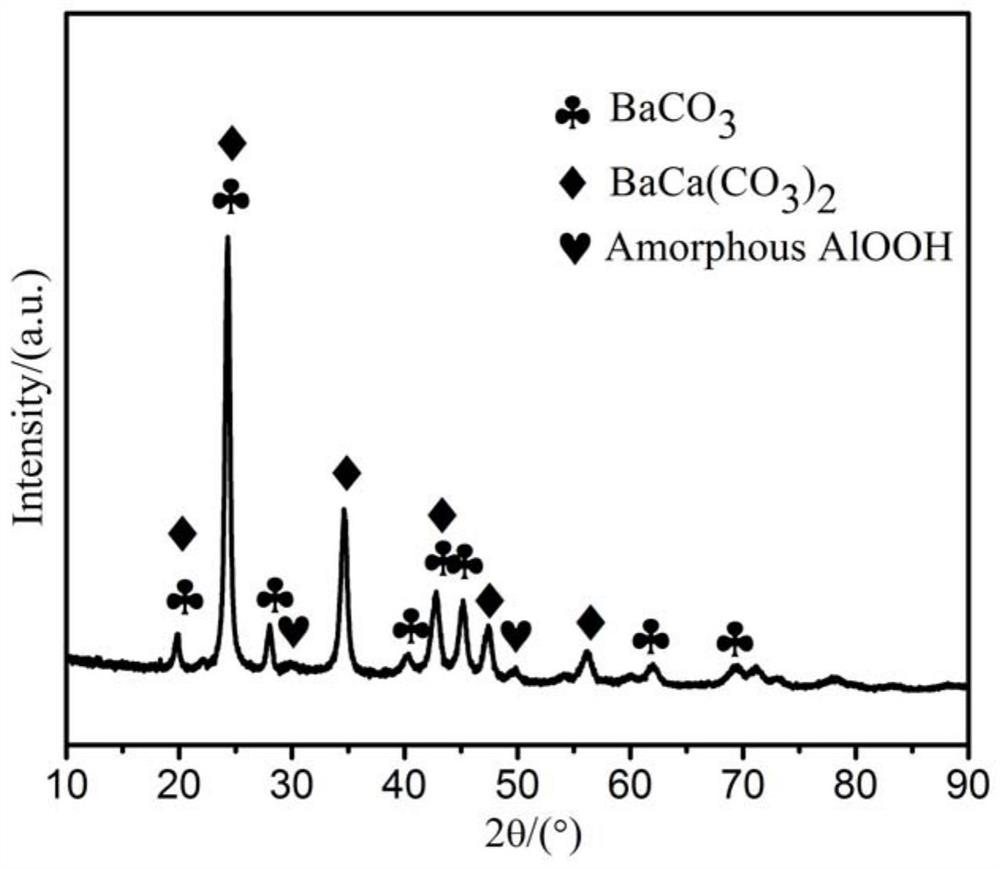
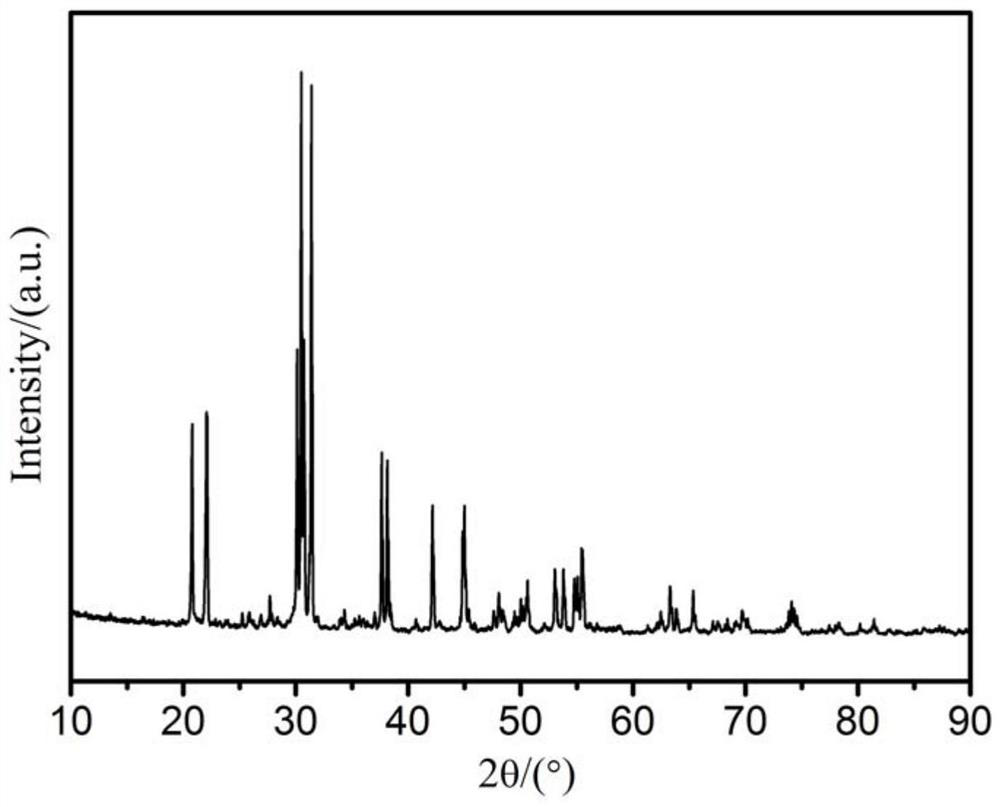
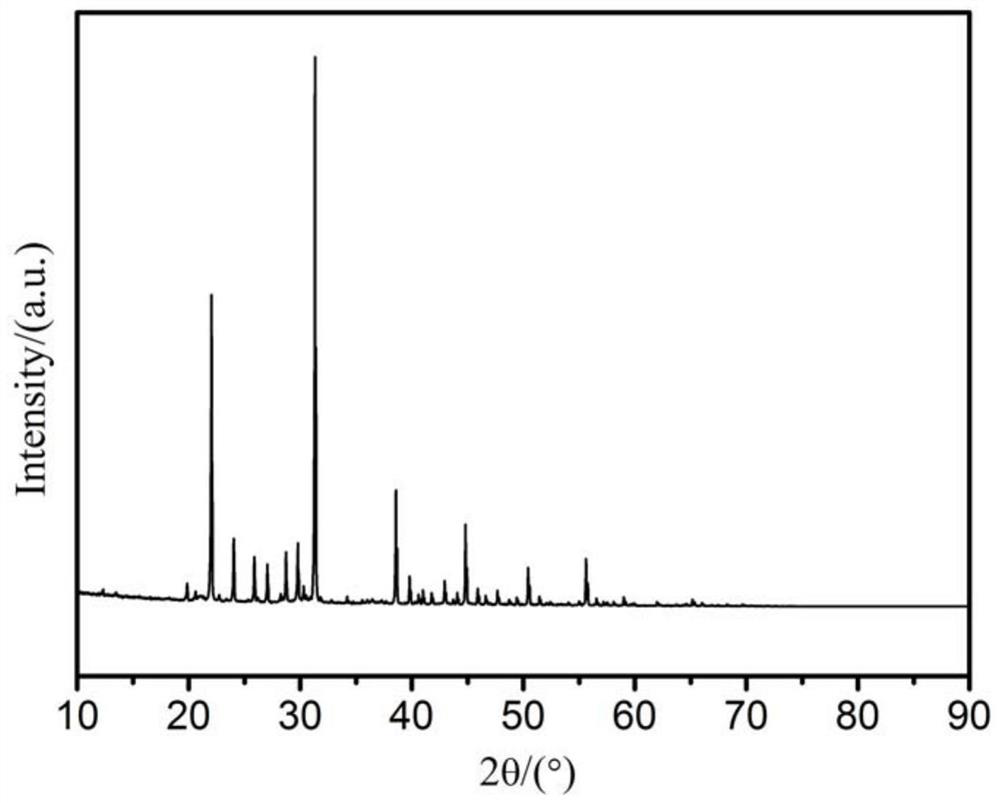
Preparation method of aluminate electron emission material for impregnated cathode
An electron emission material, aluminate technology, applied in the preparation of alkaline earth metal aluminate/alumina/aluminum hydroxide, circuits, electrical components, etc., can solve the problem that the chemical composition of aluminate cannot be designed to keep the composition consistent, and synthesize aluminum Solve the problems that the consistency of salt composition and performance cannot be guaranteed, and achieve the effects of precise control of chemical composition, small particle size, and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The chemically pure barium nitrate and deionized water purchased from Xilong Chemical Co., Ltd. were prepared into a barium nitrate solution with a mass concentration of 4%. After weighing a certain weight, pour it into a 255.15-gram calcined at 1300°C to constant weight Crucible (alumina content is 99.3%), weigh the solution and the accurate mass of the crucible is 411.15 grams, first put the crucible in an oven at 100°C for 24 hours, then place it in an electric furnace and calcinate at 200°C to constant weight, weigh and calcinate The exact weight of the product, together with the crucible, was 261.39 grams, resulting in a weight of 6.24 grams of calcined barium nitrate. The chemically pure calcium nitrate purchased from Xilong Chemical Co., Ltd. and deionized water were prepared into a calcium nitrate solution with a mass concentration of 20%. After weighing a certain weight, pour it into a calcined at 1300°C to a constant weight, weighing 263.02 grams. Crucible (al...
Embodiment 2
[0032] The chemically pure barium nitrate and deionized water purchased from Sinopharm Group Chemical Reagent Co., Ltd. were prepared into a barium nitrate solution with a mass concentration of 12%. After a certain weight was weighed, it was poured into a 269.39 g barium nitrate solution calcined at 1400°C to a constant weight. Crucible (alumina content is 99.5%), weigh the solution and the accurate mass of the crucible is 421.45 grams, first place the crucible in an oven at 150°C for 12 hours, then place it in an electric furnace and calcinate at 500°C until constant weight, weigh the calcined The exact weight of the product, together with the crucible, was 287.64 grams, resulting in a weight of 18.25 grams of calcined barium nitrate. The chemically pure calcium nitrate purchased from Sinopharm Group Chemical Reagent Co., Ltd. and deionized water were prepared into a calcium nitrate solution with a mass concentration of 45%. After a certain weight was weighed, it was poured in...
Embodiment 3
[0039] The chemically pure barium nitrate purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. and deionized water were prepared into a barium nitrate solution with a mass concentration of 8%, weighed a certain weight, poured it into calcination at 1400°C to constant weight, and the weight was 258.35 gram of crucible (aluminum oxide content is 99.7%), weigh the solution and the accurate mass of the crucible is 410.28 grams, first put the crucible in a 120°C oven and bake it for 18 hours, then place it in an electric furnace and calcinate it to constant weight at 400°C, weigh Measure the calcined product together with the accurate weight of the crucible of 270.50 grams to obtain a weight of 12.15 grams of calcined barium nitrate. The chemically pure calcium nitrate purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. and deionized water were prepared into a calcium nitrate solution with a mass concentration of 30%. Weighed a certain weight and poured it i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com