Novel preparation method of p-aminobenzamidine hydrochloride
A technology of aminobenzamidine hydrochloride and acetamidobenzonitrile, which is applied in the field of preparation of p-aminobenzamidine hydrochloride, can solve the problems of operator's health impact, strong equipment corrosion, low reaction efficiency and the like, and achieves The effect of short reaction time, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
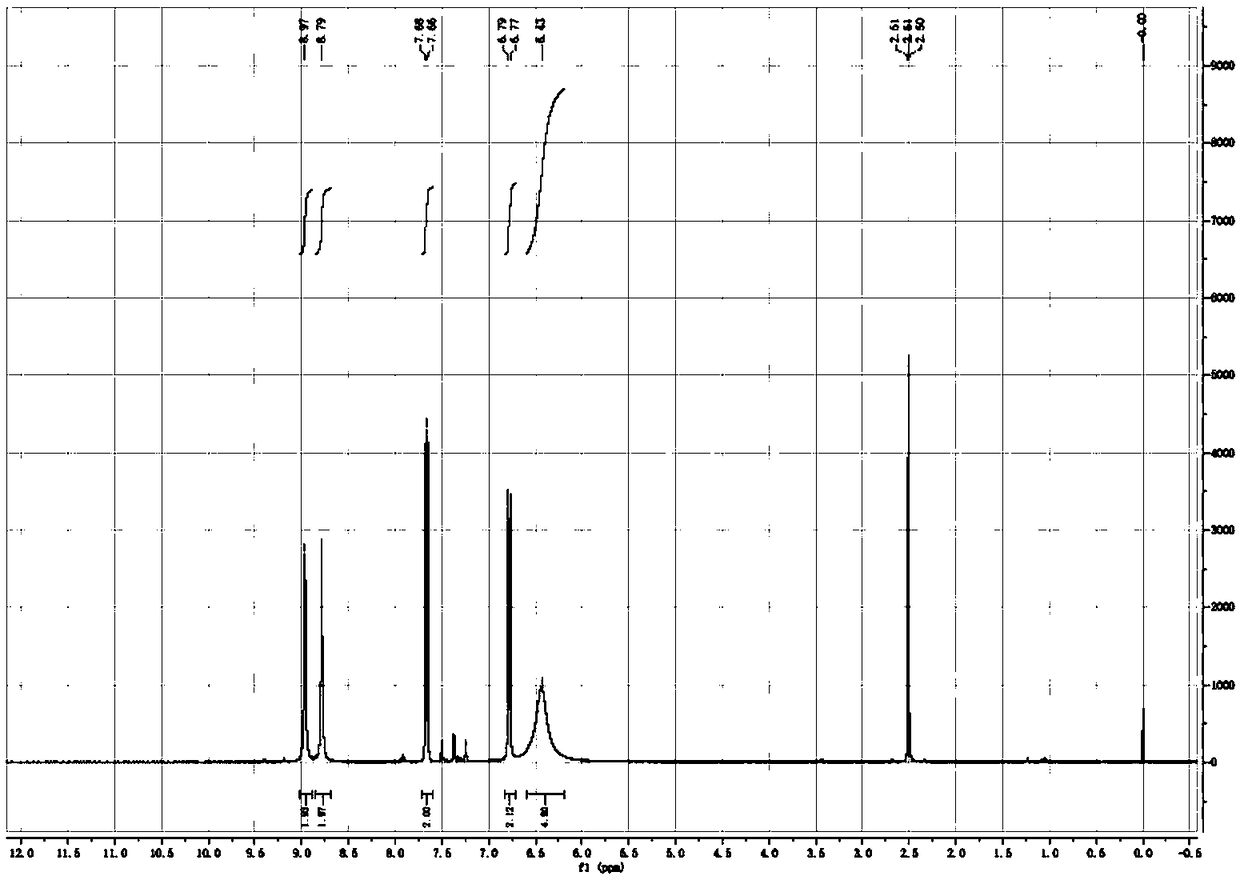
[0033] Dissolve 1 kg of p-acetamidobenzonitrile in 5 L of dimethyl sulfoxide, add 853 g of sodium amide in batches, stir at room temperature for 30 minutes, then raise the temperature to 80° C. and continue the reaction for 1 hour, monitored by TLC (developing solvent: ethyl acetate / Petroleum ether (60-90°C) = 1 / 1 (V / V)) until p-acetamidobenzonitrile disappears. Cool the reaction solution to 0°C, add concentrated hydrochloric acid dropwise under rapid stirring until the system pH=3-4, stir for 30 minutes, add 5L absolute ethanol to the reaction solution, stir for 1 hour, add the reaction solution to 10L ethyl acetate Stir in the ester for 1 hour at room temperature, filter, and dry the filter cake under reduced pressure at 40°C for 4 hours to obtain 1.27 kg of p-aminobenzonitrile hydrochloride as a white solid with a yield of 97.8% and a purity of 99.6%. 1 H-NMR (DMSO-d 6 ,δ TMS 0): 8.97ppm (2H, s, heavy water exchange disappears), 8.79ppm (2H, s, heavy water exchange disap...
Embodiment 2
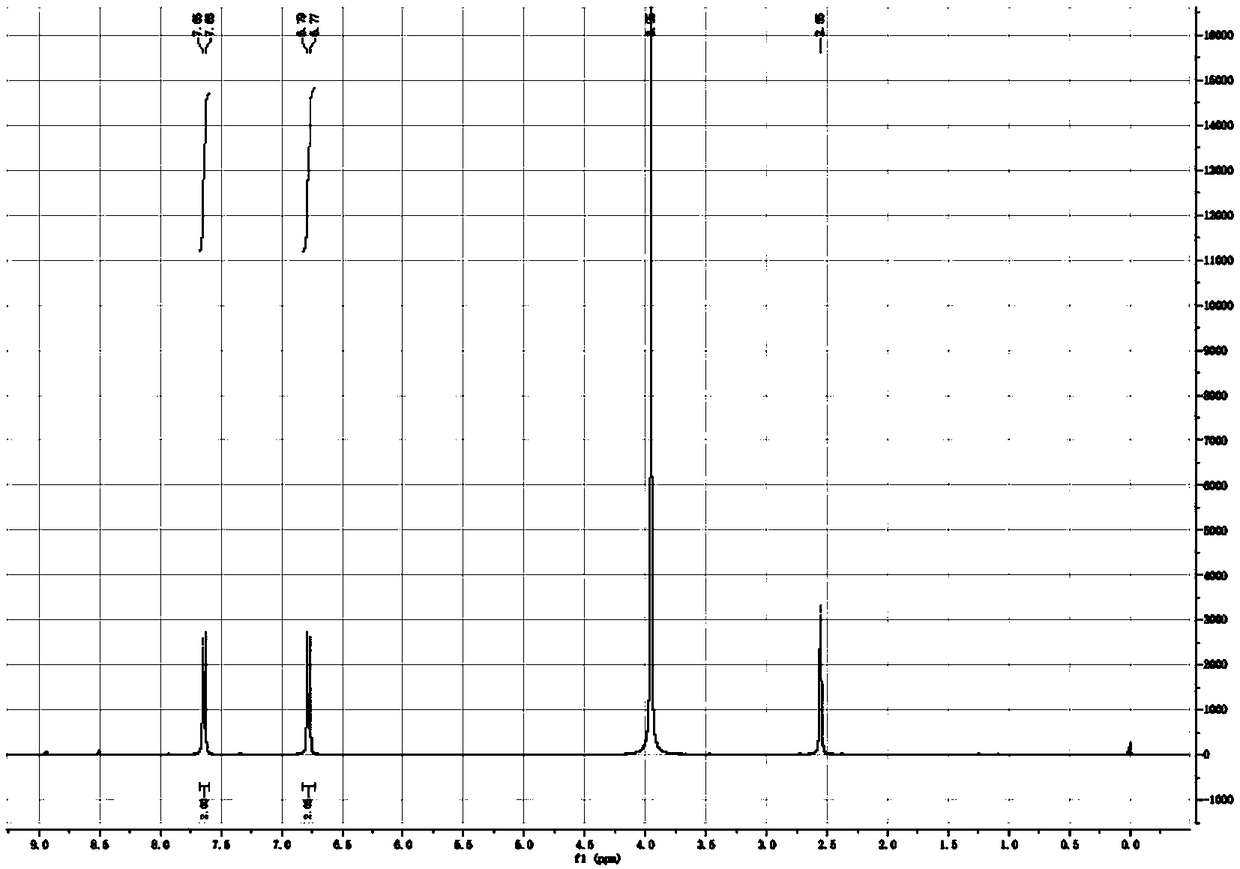
[0035] Dissolve 500g of p-acetamidobenzonitrile in 2L of tetrahydrofuran, slowly add 1.56L of sodium bis(trimethylsilyl)amide (2M in THF), stir at room temperature for 30 minutes, then raise the temperature to 80°C and continue the reaction for 2 hours, TLC Monitor (developer: ethyl acetate / petroleum ether (60-90°C) = 1 / 1 (V / V)) until p-acetamidobenzonitrile disappears. Cool the reaction solution to 0°C, add concentrated hydrochloric acid dropwise under rapid stirring until the system pH=3-4, after stirring for 1 hour, add 2L absolute ethanol to the reaction solution, stir for 1 hour, add the reaction solution to 4L ethyl acetate Stir in the ester for 1 h at room temperature, filter, and dry the filter cake under reduced pressure at 40°C for 4 hours to obtain 625.9 g of p-aminobenzonitrile hydrochloride as a white solid with a yield of 96.3% and a purity of 98.2%. The NMR and mass spectrometry identification results are consistent with Example 1.
Embodiment 3
[0037] Dissolve 20g of p-acetamidobenzonitrile in 100mL of toluene, slowly add 17g of sodium amide, stir at room temperature for 30 minutes, then raise the temperature to 80°C and continue the reaction for 4 hours, TLC monitoring (developing solvent: ethyl acetate / petroleum ether (60~ 90°C)=1 / 1(V / V)) until the p-acetamidobenzonitrile disappears. Cool the reaction solution to 0°C, add concentrated hydrochloric acid dropwise under rapid stirring until the system pH=3-4, stir for 1 hour, add 100 mL of absolute ethanol to the reaction solution, stir for 1 hour, add the reaction solution to 160 mL of ethyl acetate Stir in the ester for 1 h at room temperature, filter, and dry the filter cake under reduced pressure at 40°C for 4 hours to obtain 22.8 g of p-aminobenzonitrile hydrochloride as a white solid with a yield of 87.7% and a purity of 98.9%. The NMR and mass spectrometry identification results are consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com