A kind of dithienylethylene compound with tetraphenylethylene unit and its preparation method and application
A technology of dithienylethylene and tetraphenylethylene, applied in chemical instruments and methods, organic chemistry, instruments, etc., to achieve the effect of cheap and easy-to-obtain raw materials, rich functions, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of compound shown in formula (I), synthetic route is as follows:
[0048]
[0049] THF in the reaction formula is tetrahydrofuran.
[0050] Concrete synthetic steps are as follows:
[0051] Under the protection of nitrogen, tetraphenylethyleneaniline (0.5 g, 1.29 mmol), 30 mL of anhydrous tetrahydrofuran and 1 mL of triethylamine were sequentially added into a 100 mL reaction flask. Under the condition of ice-water bath, 1,2-bis(5-methylthiophene-2-yl chloride)cyclopentene (0.25g, 0.65mmol) in tetrahydrofuran was slowly added dropwise into the reaction system, and reacted overnight at 60°C. After the reaction, cool to room temperature, extract three times with dichloromethane, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and separate the light yellow solid through column chromatography, which is the formula ( The compound shown in I) has a yield of 43%.
[0052] ...
Embodiment 2
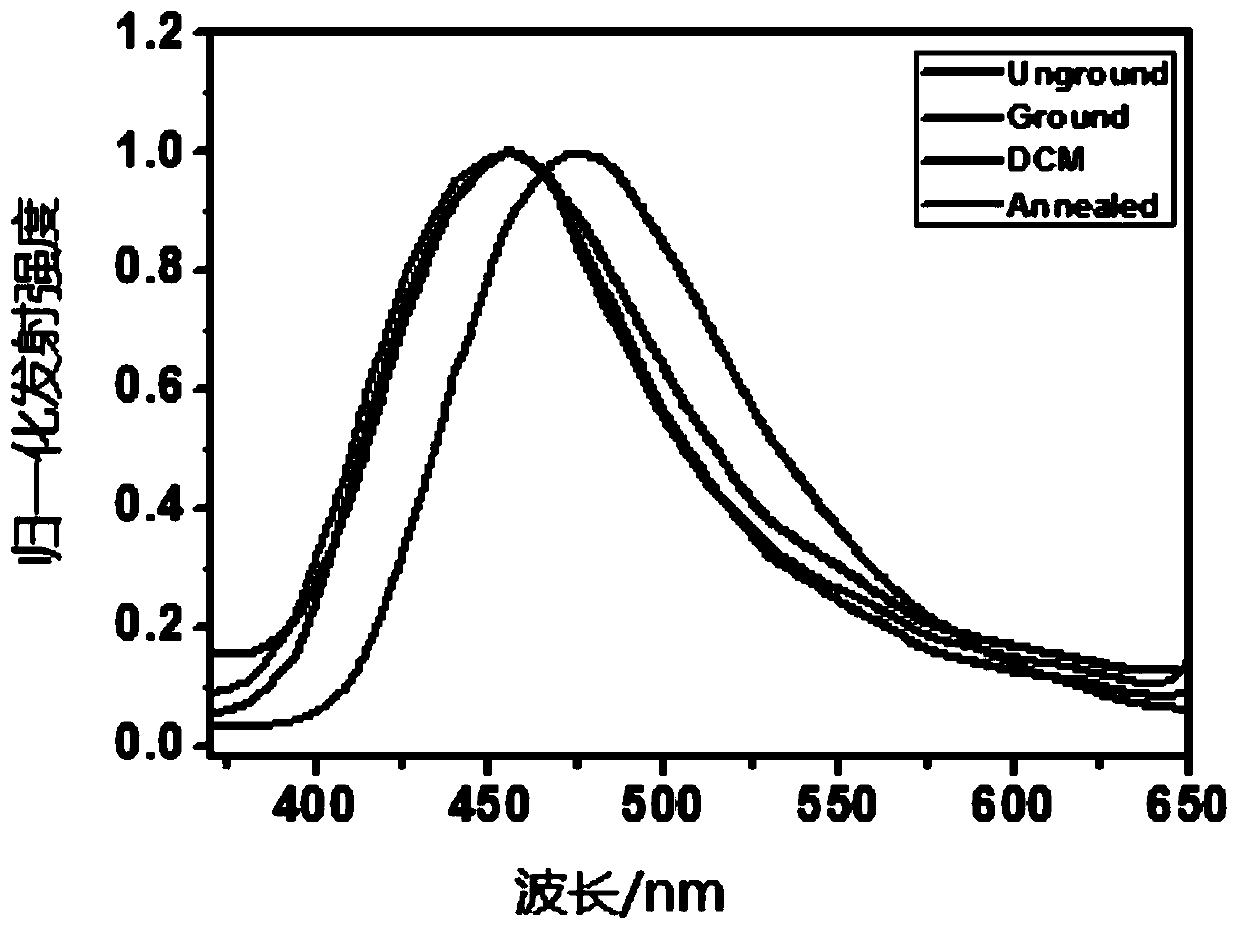
[0054] Reversible mechanochromic properties of compounds
[0055] The compound of Example 1 was processed and tested in the following different ways.
[0056] 1) Take the solid of the compound prepared in Example 1, and use 330nm as excitation light to test its fluorescence emission spectrum.
[0057] 2) Put the solid of the compound prepared in Example 1 into a mortar and grind it thoroughly, and use 330nm as excitation light to test its fluorescence emission spectrum.
[0058] 3) The solid of the compound prepared in Example 1 was thoroughly ground in a mortar, and the ground solid powder was heated at 150° C. for 3 min and cooled to room temperature, and its fluorescence emission spectrum was measured with 330 nm as excitation light.
[0059] 4) Put the solid of the compound prepared in Example 1 into a mortar and grind it thoroughly, and then dissolve the ground solid powder with dichloromethane. After the solvent evaporates, use 330nm as the excitation light to test its ...
Embodiment 3
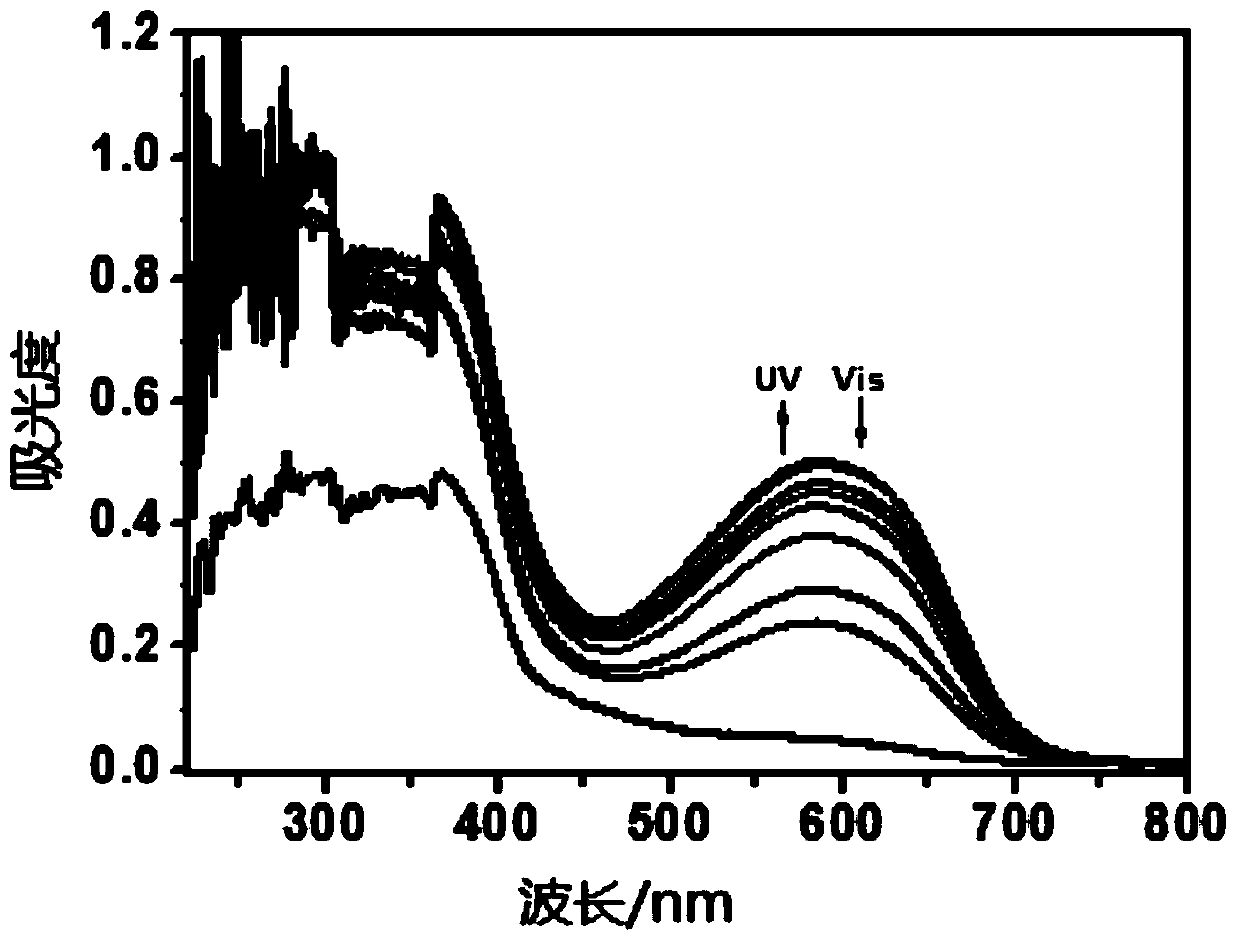
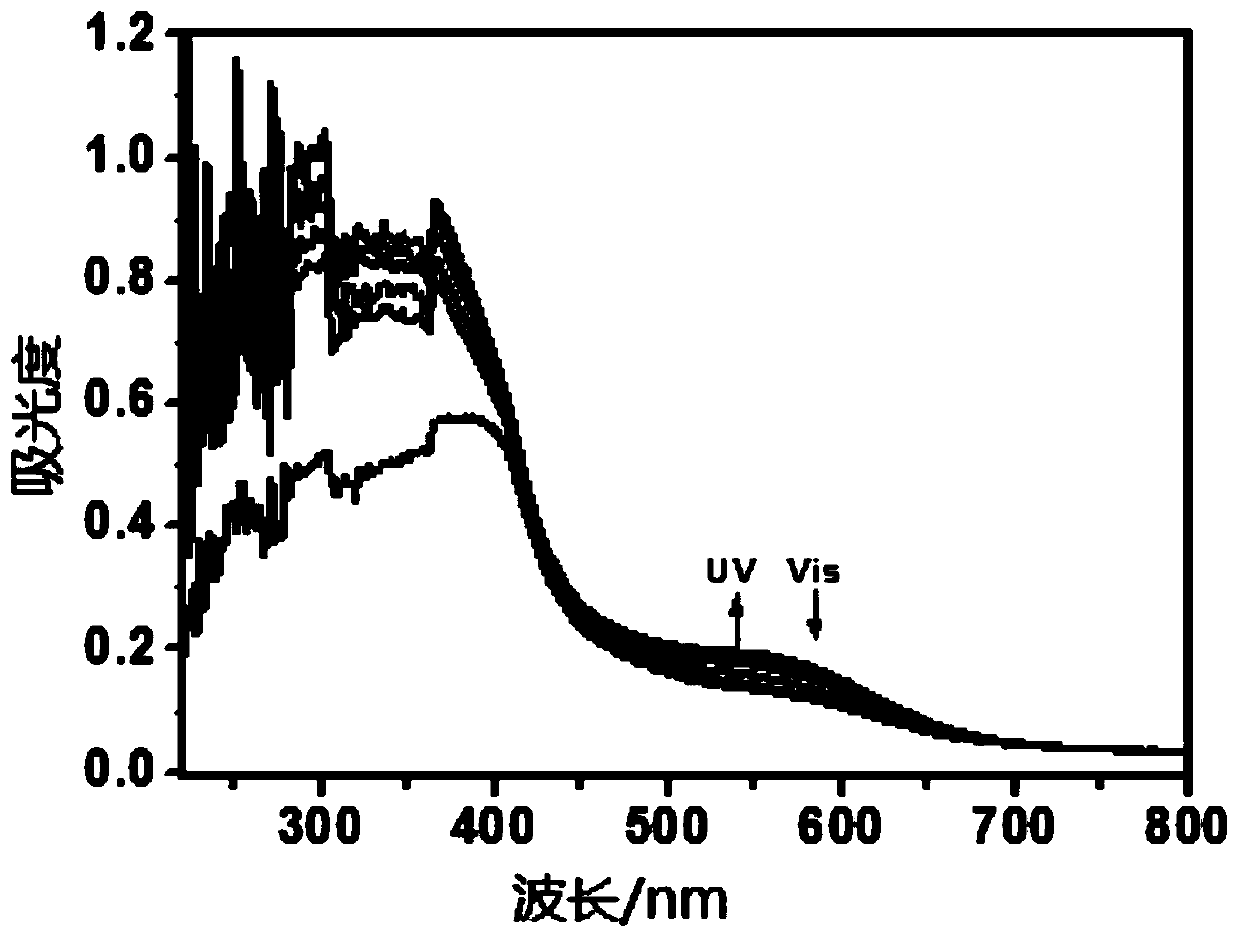
[0062] Photochromic Properties of Compound Solids
[0063] The compound of Example 1 was processed and tested in the following different ways.
[0064] 1) Get the solid of the compound prepared in Example 1, alternately irradiate with ultraviolet light and visible light of 254nm, test its ultraviolet-visible absorption spectrum, as figure 2 shown.
[0065] 2) The solid of the compound prepared in Example 1 is placed in a mortar and thoroughly ground, alternately irradiated with 254nm ultraviolet light and visible light, and tests its ultraviolet-visible absorption spectrum, such as image 3 shown.
[0066] 3) Put the solid of the compound prepared in Example 1 into a mortar and grind it thoroughly, heat the ground solid powder at 150°C for 3 minutes and cool it to room temperature, and alternately irradiate it with 254nm ultraviolet light and visible light to test its ultraviolet light. - Visible absorption spectra, such as Figure 4 shown.
[0067] Depend on figure 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com