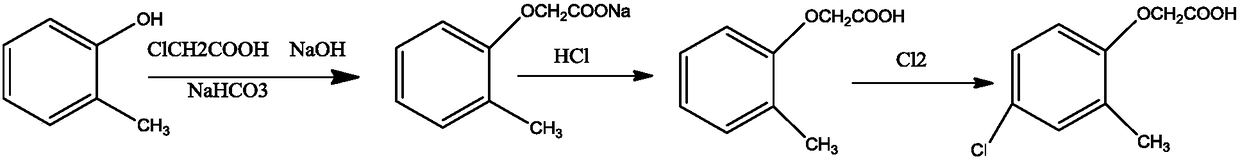
Synthesis method of 2-methyl-4-chlorophenoxyacetic acid
A technology of o-methylphenoxyacetic acid and chlorophenoxyacetic acid, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., can solve the problem of reducing product content and yield, refractory waste acid, reaction Incomplete and other problems, to achieve the effect of increasing content and yield, reducing waste acid production, and less "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
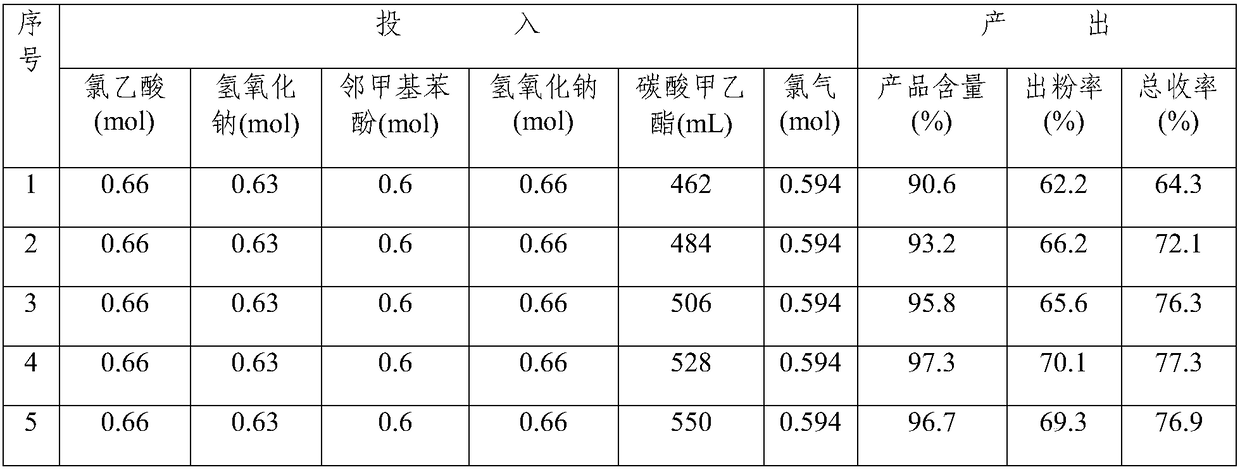
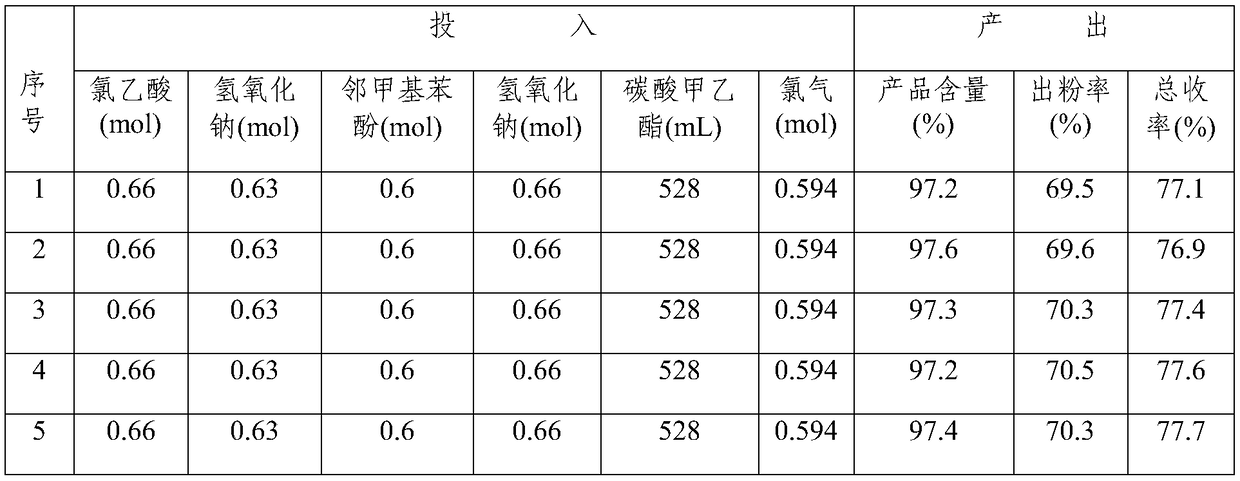
Examples
Embodiment 1
[0019] A kind of synthetic method of 2-methyl-4-chlorophenoxyacetic acid, comprises the following steps:
[0020] (1) Preparation of sodium chloroacetate: add quantitative chloroacetic acid and sodium bicarbonate to a special reactor, mix and stir for 2 hours, and simultaneously use 25°C water for circulating heating, and add water after sampling to completely dissolve as the reaction end point;
[0021] (2) Preparation of o-methylphenoxyacetic acid: add o-cresol in a special reactor, put it into the reaction kettle together with sodium chloroacetate under stirring and heat up to reflux, add dropwise 32% sodium hydroxide solution, and Make sure the pH value of the solution is 8-9. After the dropwise addition, continue to remove phenolic water until the temperature rises to 120°C, then start to cool down and add water, and then add hydrochloric acid dropwise to adjust the pH of the solution. After the acid adjustment is completed, suction filter and dry the filter cake. o-methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com