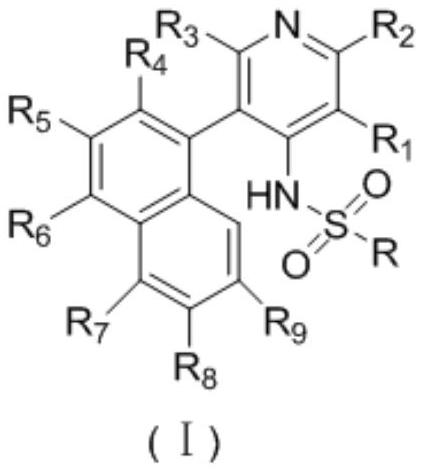
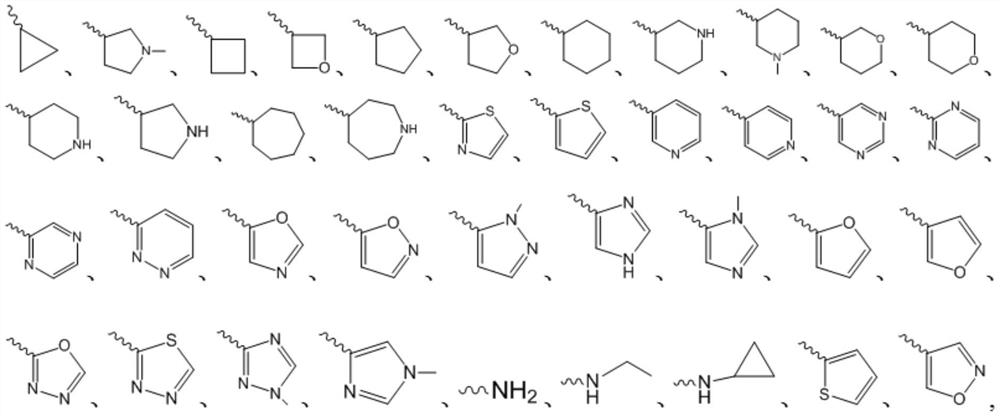
Sulfonamide compound for treating gout or hyperuricemia and preparation method thereof
A technology for hyperuricemia and compounds, applied in the field of sulfonamide compounds and their preparation, in the field of treating gout or hyperuricemia, can solve the problems of poor curative effect and increasing the response rate of gout patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The synthesis of embodiment 1,4-(4-aminopyridin-3-yl)-1-naphthalene nitrile (Int 2)
[0054] 1) Synthesis of 4-(pinacol borate-3-yl)-1-naphthonitrile (Int1)
[0055]
[0056] Add 4-bromo-1-naphthonitrile (232mg, 1mmol), potassium acetate (196mg, 2mmol), bipinacol borate (380mg, 1.5mmol), Pd(dppf)Cl to a 100mL reaction flask 2 (73mg, 0.1mmol), DMSO (5mL), nitrogen replacement three times, heated to 80°C and stirred for 6 hours. Then the reaction solution was poured into water (30mL), extracted three times with ethyl acetate (3×15ml), the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and passed through the column after rotary evaporation to obtain intermediate 1 (176mg ), yield 63%. 1 H NMR (400MHz, Chloroform-d) δ8.88–8.72(m,1H),8.30–8.18(m,1H),8.08(d,J=7.1Hz,1H),7.88(d,J=7.2Hz, 1H),7.72–7.56(m,2H),1.43(s,12H).
[0057] 2) Synthesis of 4-(4-aminopyridin-3-yl)-1-naphthonitrile (Int 2)
[0058]
[0059]...
Embodiment 2
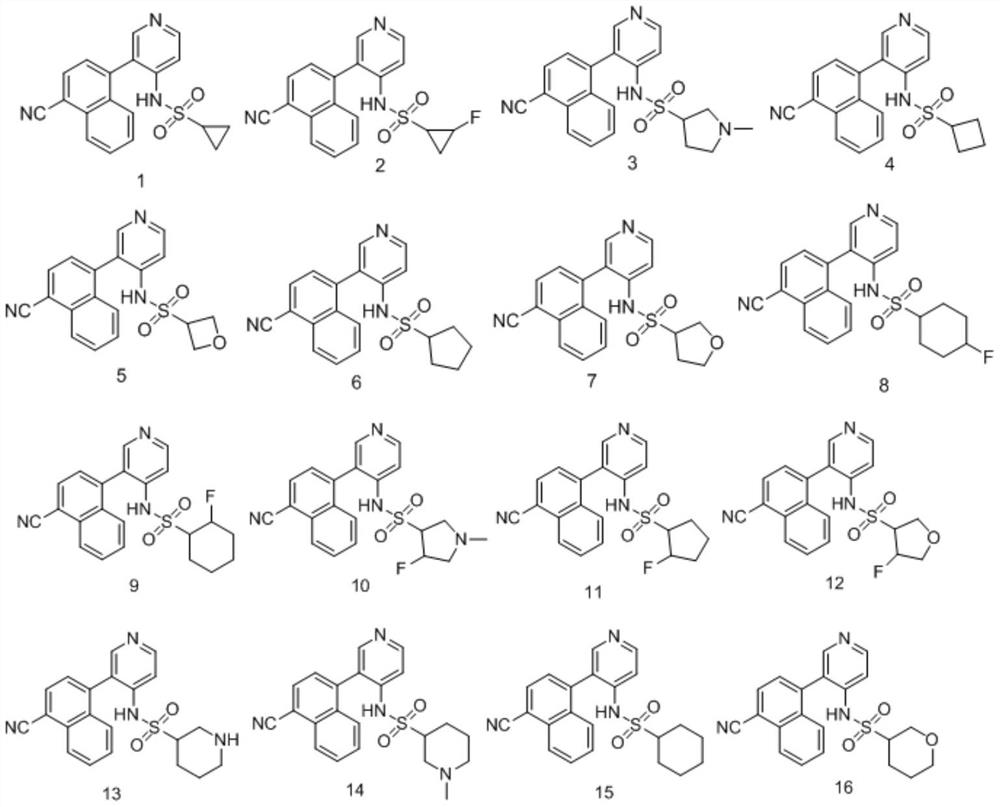
[0061] Embodiment 2, the synthesis of N-(3-(4-cyanonaphthalene-1-yl)pyridin-4-yl)cyclobutylsulfonamide (4)
[0062]
[0063] Add Intermediate 2 (245mg, 1mmol), dichloromethane (8mL), triethylamine (303mg, 3mmol) to a 100mL reaction flask, and add cyclobutylsulfonyl chloride (185mg, 1.2mmol) in batches under ice-water bath conditions ), naturally slowly return to room temperature and react for 3 hours. After the reaction was completed, the reaction solution was poured into water (30mL), extracted three times with dichloromethane (3×15ml), the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and passed through the column after rotary evaporation to obtain compound 4 (72 mg), yield 20%. Mass Spectrum: 364.1(M+H + ).
[0064] 1 H NMR (400MHz, Chloroform-d) δ8.74–8.66 (m, 1H), 8.49 (d, J = 5.1Hz, 1H), 8.41 (dd, J = 8.4, 5.2Hz, 1H), 8.23 (dd, J=6.9,5.4Hz,1H),8.11(dd,J=7.4,5.4Hz,1H),7.90–7.80(m,1H),7.77–7.68(m,1H),7.6...
Embodiment 3
[0065] Example 3, N-(3-(4-cyanonaphthalene-1-yl)pyridin-4-yl)cyclopropylsulfonamide (1)
[0066]
[0067] Replace the raw material cyclobutylsulfonyl chloride for the preparation of compound 4 with cyclopropylsulfonyl chloride, and follow the same preparation method as compound 4 to obtain compound 1, mass spectrum: 350.1 (M+H + ).
[0068] 1H NMR (400MHz, Chloroform-d) δ8.74–8.66 (m, 1H), 8.49 (d, J = 5.1Hz, 1H), 8.41 (dd, J = 8.4, 5.2Hz, 1H), 8.23 (dd, J=6.9,5.4Hz,1H),8.11(dd,J=7.4,5.4Hz,1H),7.90–7.80(m,1H),7.77–7.68(m,1H),7.65(dd,J=7.4, 5.3Hz, 1H), 7.58(dd, J=8.5, 5.2Hz, 1H), 7.37(s, 1H), 2.74–2.81(m, 1H), 1.39–1.13(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com