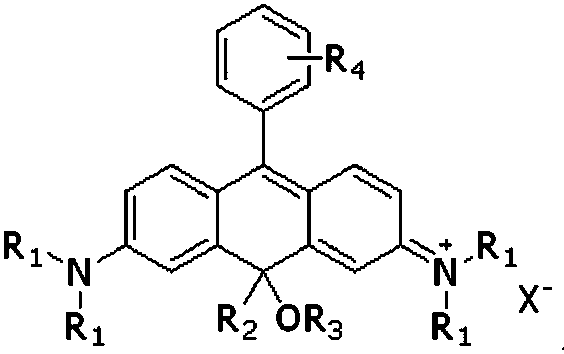
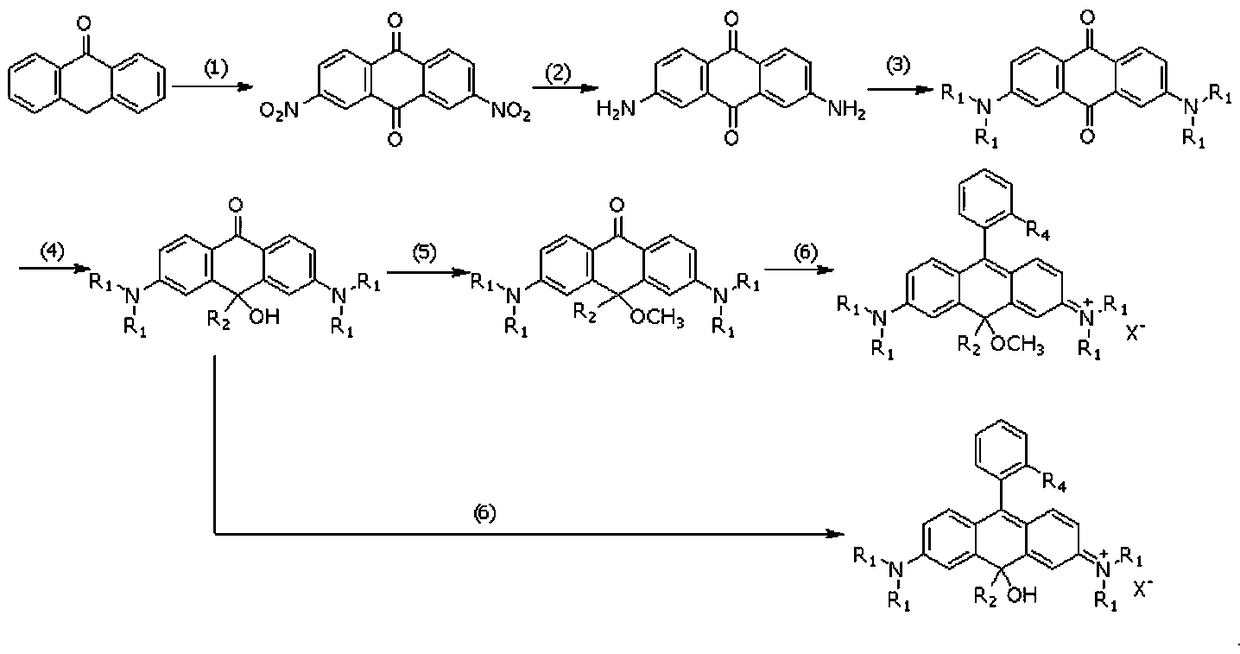
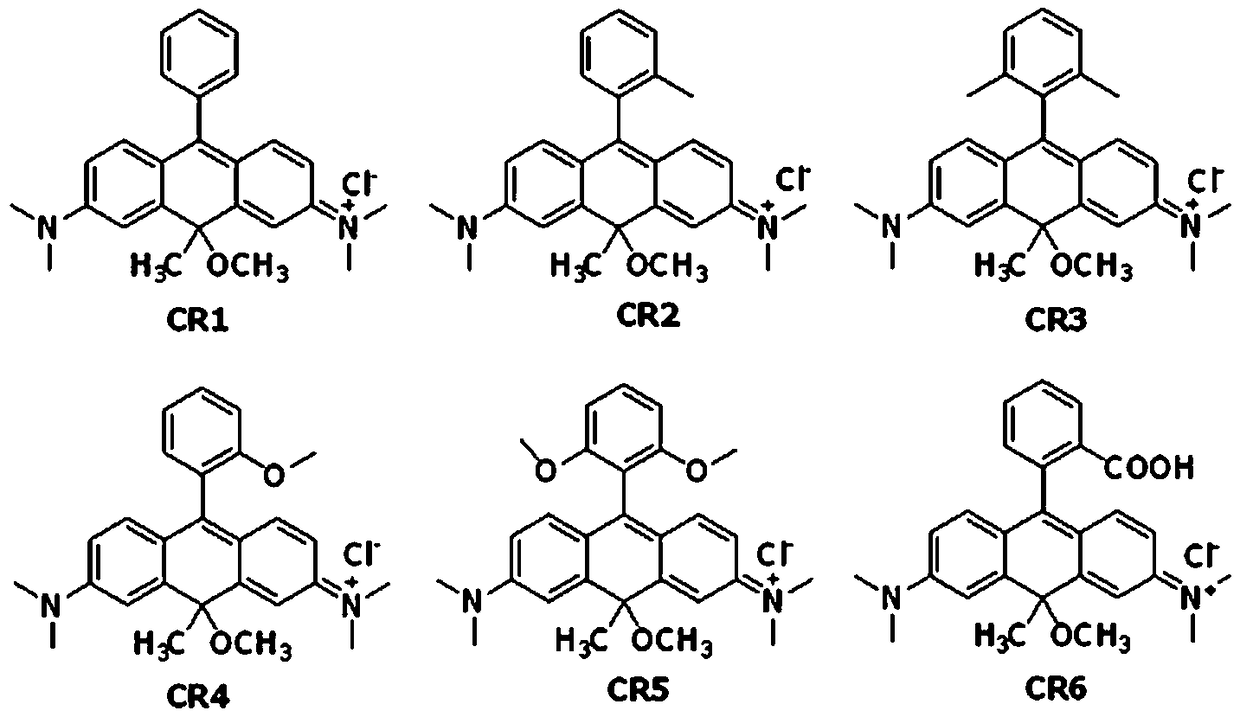
Near-infrared carbon rhodamine fluorescent dye and synthetic method thereof
A technology for fluorescent dyes and synthesis methods, which is applied in the field of near-infrared carborhodamine fluorescent dyes and their synthesis, can solve the problems of difficult synthesis of carborhodamine, and achieve the effects of good water solubility, high quantum yield, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1, the synthesis of near-infrared carborhodamine fluorescent dye CR1, using intermediate 5-1 to synthesize CR1.
[0048] The first step, the synthesis of 2,7-dinitroanthracene-9,10-dione
[0049] Dissolve anthrone (3.90 g, 20 mmol) in 25 ml of fuming nitric acid in an ice bath at 0-5°C, stir for 2 hours, and continue the reaction for 2 hours after rising to room temperature, then pour into 70 ml of glacial acetic acid, and statically After standing overnight, a bright yellow precipitate precipitated out. After suction filtration, the filter cake was recrystallized with glacial acetic acid to obtain light yellow solid compound 2,7-dinitroanthracene-9,10-dione (2.98 g, 10 mmol), with a yield of 50%. 1 H-NMR (DMSO-d 6 , 600MHz) δ (ppm): 8.84 (d, J=2.4Hz, 1H); 8.71 (d, J = 2.4Hz, 2H); 8.49 (d, J = 9.0 Hz, 2H).
[0050] The second step, the synthesis of 2,7-diaminoanthracene-9,10-dione
[0051] Sodium hydroxide (2.15 g, 53.5 mmol) was dissolved in 80 ml of water,...
Embodiment 2
[0061] Example 2, the synthesis of near-infrared carborhodamine fluorescent dye CR7, using intermediate 4-1 to synthesize CR7.
[0062] The first step to the fourth step are the same as in Example 1.
[0063] The fifth step, the synthesis of near-infrared carborhodamine fluorescent dye CR7
[0064] Dissolve 2-methylbromobenzene (0.51 g, 3 mmol) in 10 ml of dry tetrahydrofuran, cool to -78°C in an acetone liquid nitrogen bath, and then add n-butyllithium solution (2.4 mol / L THF solution, 0.5 ml), and stirred for 1 hour. Add 2,7-bis(dimethylamino)-9-hydroxy-9-methyl-anthracen-10-one (93 mg, 0.3 mmol) in tetrahydrofuran (5 ml) dropwise, and slowly return to room temperature (25 ℃), reacted for 2 hours, and then acidified with 2 mol / L dilute hydrochloric acid, the solution changed from yellow to blue-green. The reaction solution was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was spin-dried, an...
Embodiment 3
[0066] Example 3, the synthesis of near-infrared carborhodamine fluorescent dye CR9, using intermediate 4-2 to synthesize CR9.
[0067] The first step to the third step are the same as in Example 1.
[0068] The fourth step, the synthesis of 2,7-bis(dimethylamino)-9-hydroxy-9-phenyl-anthracene-10-one (intermediate 4-2)
[0069] Dissolve 2,7-bis(dimethylamino)anthracene-9,10-dione (0.10 g, 0.33 mmol) in 10 ml of dry tetrahydrofuran, then add phenylmagnesium bromide (1mol / L THF solution, 1 ml), the reaction was stirred at room temperature for 30 minutes, and then quenched with dilute hydrochloric acid solution. Add 50 ml of water to the reaction solution, extract 3 times with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, spin to dry the solvent, and separate the yellow solid compound 2,7-bis(dimethylamino )-9-hydroxy-9-phenyl-anthracen-10-one (80 mg, 0.23 mmol), yield 70%. 1 H-NMR (CDCl 3 , 600MHz) δ (ppm): 8.19 (d, J=9.0Hz, 2H); 7.38 (s, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com