A kind of indole methyl aniline compound and application thereof
A kind of technology of indole methyl aniline and compound, which is applied in the field of substituted 4-methyl)-N-methyl aniline compounds, can solve the problems such as structure and biological activity that have not been reported in literature and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
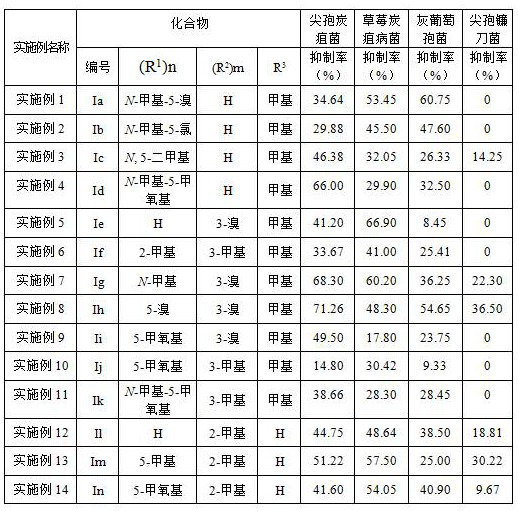
[0027] Embodiment 1 Derivative Ia ((R 1 )n = N -Methyl-5-bromo, (R 2 )m = H, R 3 = methyl) synthesis
[0028] weigh N -Methyl-5-bromoindole (0.5 mmol), N , N -Dimethylaniline (4.0 eq.) and 1.5 mol% of RoseBengal were placed in a 25 mL Schlenk reaction tube, and then 5 mL of tetrahydrofuran was added, under the state of air, placed under a 15 W LED white light to react, and stirred at room temperature for the reaction, After 24 h, the reaction was completed, the solvent was removed, and the concentrated solution was separated by column chromatography (petroleum ether / ethyl acetate=15:1, V / V) to obtain a yellow oily liquid, namely derivative Ia. Yield 61%.
[0029] of the compound 1 H NMR, 1 C NMR and ESI-HRMS analysis data as follows,
[0030] 1 H NMR (CDCl 3 , 500 MHz) δ 7.74 (d, J = 2.0 Hz, 1H), 7.33 (dd, J = 8.5,2.0 Hz, 1H), 7.20 (d, J = 8.5, 2 H), 7.17 (d, J = 9.0 Hz, 1 H), 6.77 (s, 2H), 6.76 (t, J = 2.5 Hz, 1H), 4.01 (s, 2H), 3.71 (s, 3H), 2.98 (s, 6H...
Embodiment 2
[0031] Embodiment 2 Derivatives Ib ((R 1 )n = N -Methyl-5-chloro, (R 2 )m = H, R 3 = methyl) synthesis
[0032] weigh N -Methyl-5-chloroindole (0.5 mmol), N , N -Dimethylaniline (5.5 eq.) and 3.5 mol% Rhodamine B were placed in a 25 mL Schlenk reaction tube, then 5 mL of chloroform was added, under the state of air, placed under a 20 WLED white light to react, and stirred at room temperature The reaction was tracked and monitored by TLC. After 36 h, the reaction was completed, the solvent was removed, and the concentrated solution was separated by column chromatography (petroleum ether / ethyl acetate=10: 1, V / V) to obtain a yellow oily liquid, namely derivative Ib. Yield 51%.
[0033] of the compound 1 H NMR, 1 C NMR and ESI-HRMS analysis data as follows,
[0034] 1 H NMR (CDCl 3 , 500 MHz) δ 7.56 (d, J = 1.0 Hz, 1H), 7.25-7.2 (m, 3H),6.18 (s, 1H), 6.79 (s, 1H), 6.75 (d, J = 9.0 Hz, 2H), 4.00 (s, 2H), 3.72 (s,3H), 2.96 (s, 6H); 13 C NMR (CDCl 3 C 18 h 20 N ...
Embodiment 3
[0035] Example 3 Derivatives Ic ((R 1 )n = N , 5-Dimethyl, (R 2 )m = H, R 3 = methyl) synthesis
[0036] weigh N , 5-Dimethylindole (0.5 mmol), N , N -Dimethylaniline (5.5 eq.) and 2.0 mol% Eosin B were placed in a 25 mL Schlenk reaction tube, then 6 mL of acetonitrile and 1 mL of water were added, and the reaction was carried out under a 15 W LED white light under the condition of air ventilation, at room temperature The reaction was stirred at lower temperature and followed and monitored by TLC. After 24 h, the reaction was completed, the solvent was removed, and the concentrated solution was separated by column chromatography (petroleum ether / ethyl acetate=10: 1, V / V) to obtain wine red oily liquid, namely derivative Object Ic. The yield is 68%.
[0037] of the compound 1 H NMR, 1 C NMR and ESI-HRMS analysis data as follows,
[0038] 1 H NMR (CDCl 3 , 500 MHz) δ 7.36 (s, 1H), 7.17-7.19 (d, J = 8.5 Hz, 3H),7.05 (dd, J = 8.0, 1.5 Hz, 1H), 6.74-6.72 (d, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com