Near-infrared fluorescent dye containing 4-dicyanomethylbenzopyran unit and its preparation method and application
A cyanomethylbenzopyran and near-infrared dye technology, which can be used in methine/polymethine dyes, styryl dyes, luminescent materials, etc., can solve problems such as interference with fluorescent imaging effects, and achieve broad application prospects , The synthesis route is simple, and the effect of increasing the emission wavelength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Compound 2
[0028] Under ice bath conditions, POCl 3 (7.83 mL, 10 eq) was dropped into DMF (7.77 mL, 11 eq), and the reaction was maintained at this temperature for 2 hours. Under ice bath conditions, compound 1 (3.5 g, 7.18 mmol) was dissolved in 2 mL of DMF, dropped into the reaction system, and reacted at room temperature overnight. After the reaction was completed, the reaction was directly poured into ice water, and the pH of the solution was adjusted to near neutrality with sodium carbonate, then extracted with EA, washed three times with water (50 mL×3), and washed three times with saturated saline (50 mL×3). The organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried through a silica gel column (PE:EA=20:1) to obtain 3.5 g of compound 2. Yield: 94%.
[0029] The compound structure determination data are as follows:
[0030] 1 H NMR (400MHz, CDCl 3 )δ9.80(s, 1H), 7.68(d, J=8.6Hz, 2H), 7.34(t...
Embodiment 2
[0033] Example 2: Preparation of Compound 4
[0034] Take compound 2 (445 mg, 1 mmol) and compound 3 (208 mg, 1 mmol) into a 100 mL round-bottomed flask, add 45 mL of toluene to dissolve under argon protection, add 0.5 mL of piperidine, 0.5 mL of acetic acid, and under argon protection at 115 ° C The reaction was heated under reflux in an oil bath for 12 hours. After the reaction was completed, it was cooled to room temperature, toluene was removed by rotary evaporation, and 417 mg of compound 4 was obtained by separation and purification on a silica gel column (PE:EA=10:1). Yield: 65%.
[0035] The structural determination data of compound 4 are as follows:
[0036] 1 H NMR (400MHz, CDCl 3 )δ8.91(d,J=8.3Hz,1H),7.78-7.70(m,1H),7.62-7.54(m,2H),7.44(t,J=8.9Hz,3H),7.34(t,J =7.8Hz,2H),7.21–7.14(m,5H),7.07(dd,J=26.8,8.5Hz,4H),6.81(s,1H),6.64(d,J=15.8Hz,1H),4.30 –4.16(m,2H),2.97(t,J=7.8Hz,2H),2.65(t,J=7.8Hz,2H),1.08–0.96(m,2H),0.07(s,9H)
[0037] 13 C NMR (101MHz, CDCl 3)δ1...
Embodiment 3
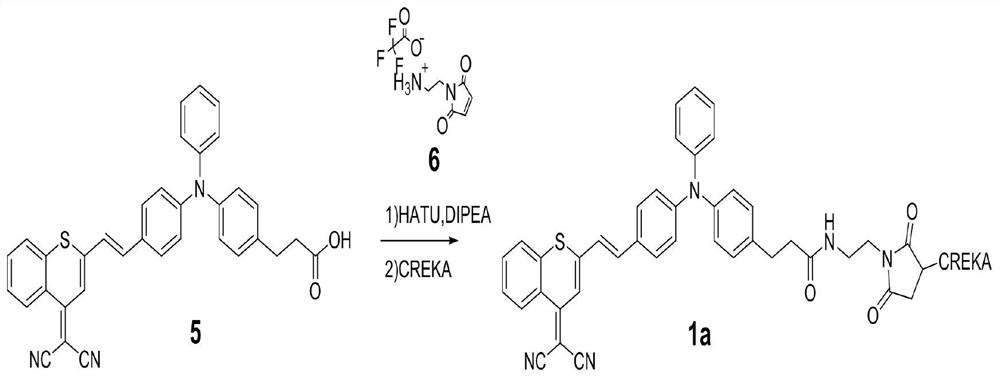
[0039] Example 3: Preparation of Compound 5
[0040] Compound 4 (20 mg, 0.03145 mmol) was taken into a 5 mL round-bottomed flask, 1 mL of dichloromethane was added under argon protection, placed in an ice bath, and 0.5 mL of trifluoroacetic acid was added dropwise. The reaction solution was mechanically stirred for 6 hours at 25°C. After the reaction was completed, the solvent was directly evaporated by rotary evaporation, and separated and purified by silica gel column (DCM:MeOH=20:1) to obtain 15 mg of compound 5 with a yield of 89%.
[0041] The structural determination data of compound 5 are as follows:
[0042] 1 H NMR (400MHz, CDCl 3 )δ8.92(dd,J=8.4,1.0Hz,1H),7.78-7.71(m,1H),7.61-7.54(m,2H),7.49-7.41(m,3H),7.34(t,J= 7.8Hz, 2H), 7.17(dd, J=15.1, 6.4Hz, 5H), 7.07(dd, J=26.3, 8.5Hz, 4H), 6.83(s, 1H), 6.66(d, J=15.8Hz, 1H), 2.99(t, J=7.7Hz, 2H), 2.74(t, J=7.7Hz, 2H).
[0043] 13 C NMR (101MHz, CDCl 3 )δ177.84,158.22,152.87,152.38,150.24,146.60,144.91,138.77,136.45,13...
PUM
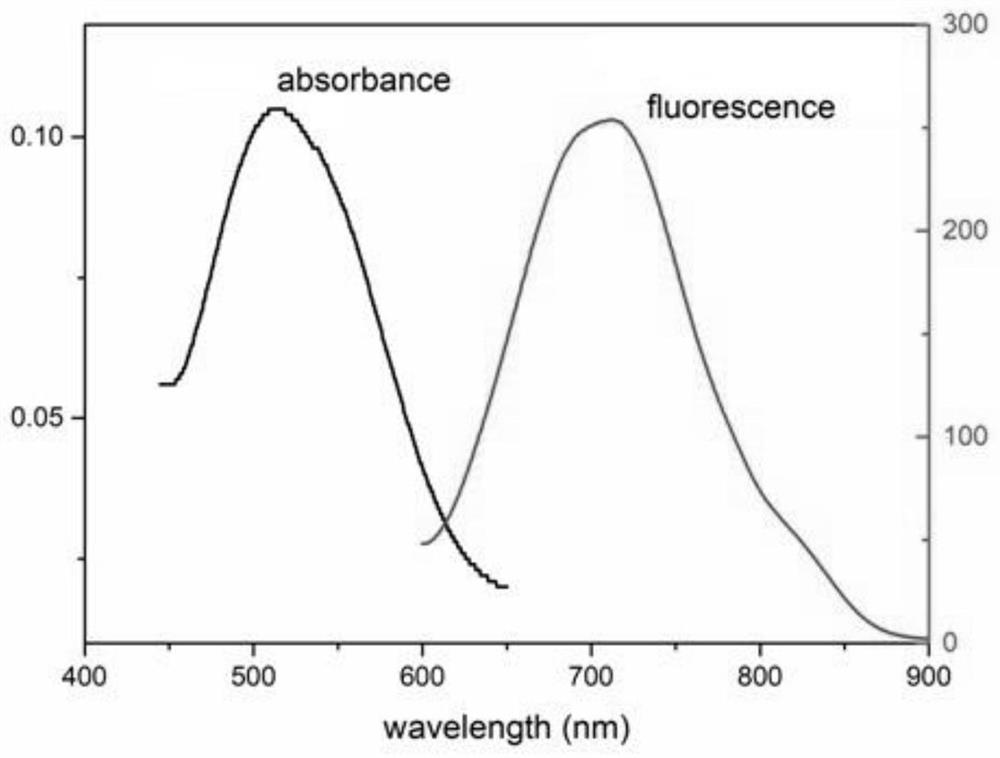
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com