Drug composition for treating HCV (Hepatitis C Virus) infection
A composition and medicine technology, applied in the field of preparing the pharmaceutical composition and unit dosage form, can solve the problems of patient treatment failure, the patient has no treatment alternatives and the like, and achieve the effect of low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
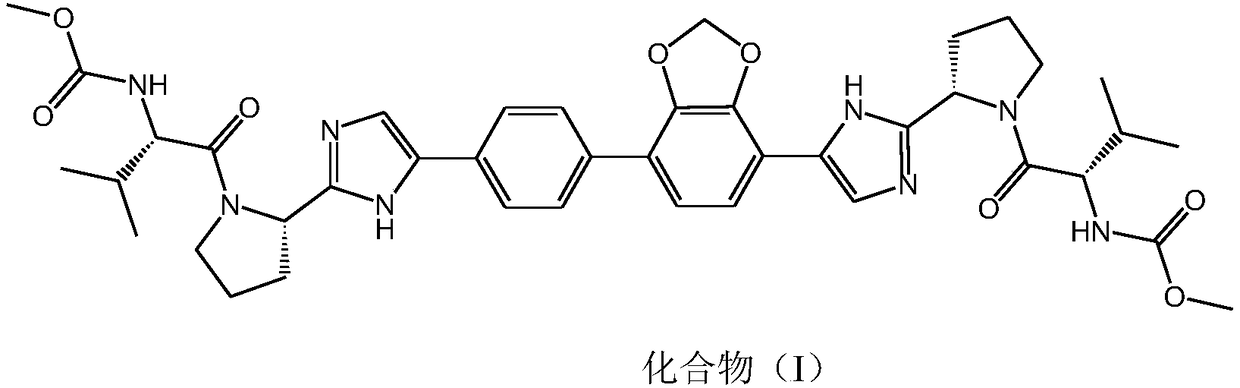
[0045] Example 1 Compound N-[(2S)-1-[(2S)-2-{4-[7-(4-{2-[(2S)-1-[(2S)-2-[(methoxy ylcarbonyl)amino]-3-methylbutyryl]pyrrolidin-2-yl]-1H-imidazol-4-yl}phenyl)-2H-1,3-benzodioxol-4-yl]- 1H-imidazol-2-yl}pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]methyl carbamate (prepared according to the method disclosed in CN102791687B) dihydrochloride salt preparation
[0046] At room temperature, a solution of pure product of structural formula I (800 g, 1.0 eq) and ethyl acetate (8 L) was successively added into a 20 L bottle and stirred. Add dropwise HCl / ethyl acetate solution (839g) with a concentration of about 11.2% into the system, control the temperature of the system at 15°C to 25°C, stir for more than 3 hours, stop the reaction, filter with suction, and filter the cake with ethyl acetate (2L) Wash, control the temperature of the filter cake and bake at 40-60°C, take a sample and test until the residue of ethyl acetate <0.5%, (about 73 hours after drying), the compound of structural...
Embodiment 2
[0047] Example 2 The compound of formula (II) is screened by different crystallization methods
[0048] 1. Slow volatile crystallization
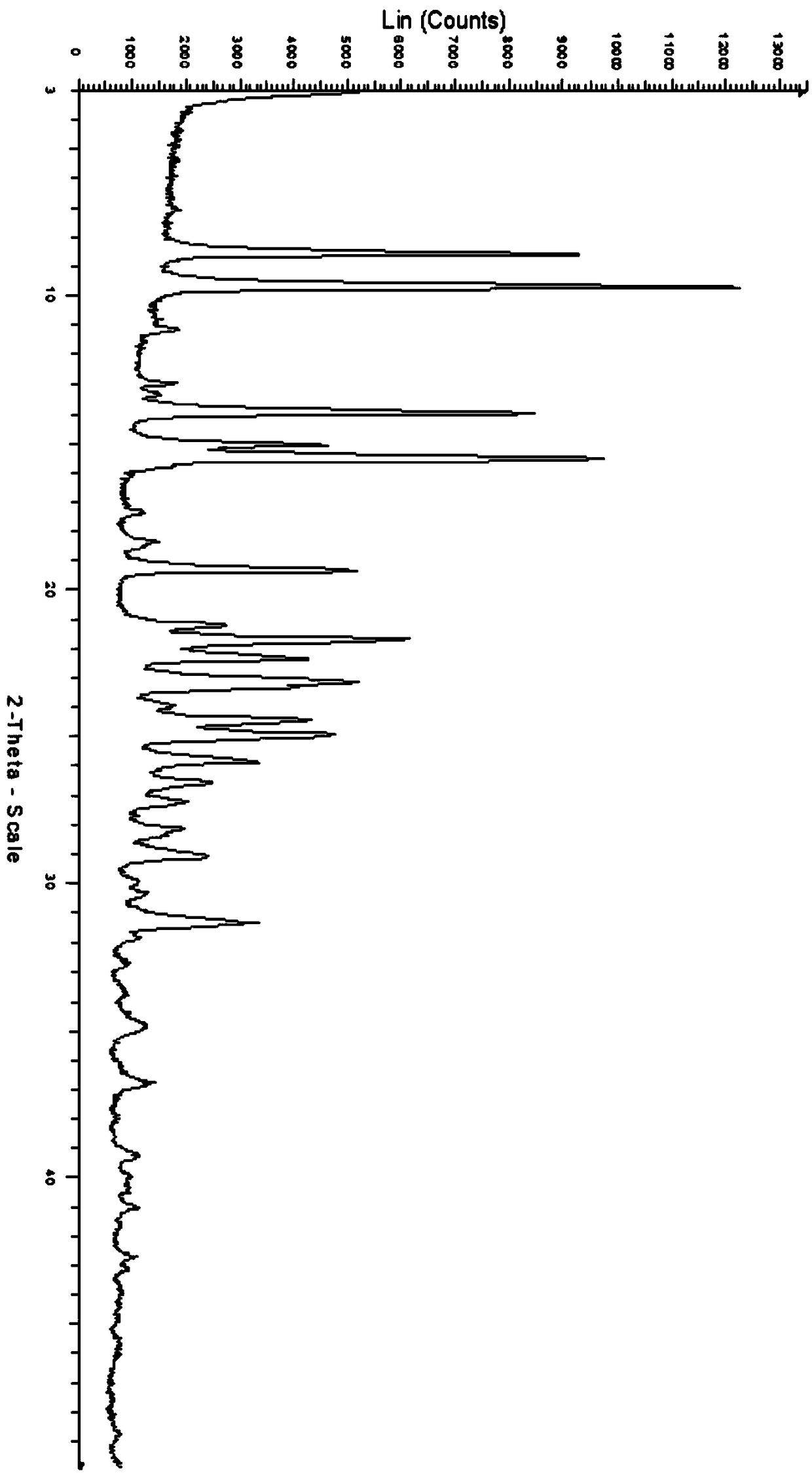
[0049] Weigh about 10mg of the pure product with structural formula II into a 3mL glass bottle, respectively add about 0.5-1.25mL of the following solvents to ensure that the samples are completely dissolved to obtain a clear solution, and the obtained solution is slowly volatilized at room temperature. Solid test XRPD, the results are shown in Table 1. The solids obtained in the volatile crystallization experiment were all amorphous, and no other new crystal forms were obtained.
[0050] Experiment number
Solvent used
temperature
get solid
805301-14-A1
H2O
RT
amorphous
805301-14-A2
MeOH
RT
amorphous
805301-14-A3
EtOH
RT
amorphous
805301-14-A4
IPA
RT
N / A*
805301-14-A5
RT
amorphous
805301-14-A6
DMSO
RT
...
Embodiment 3
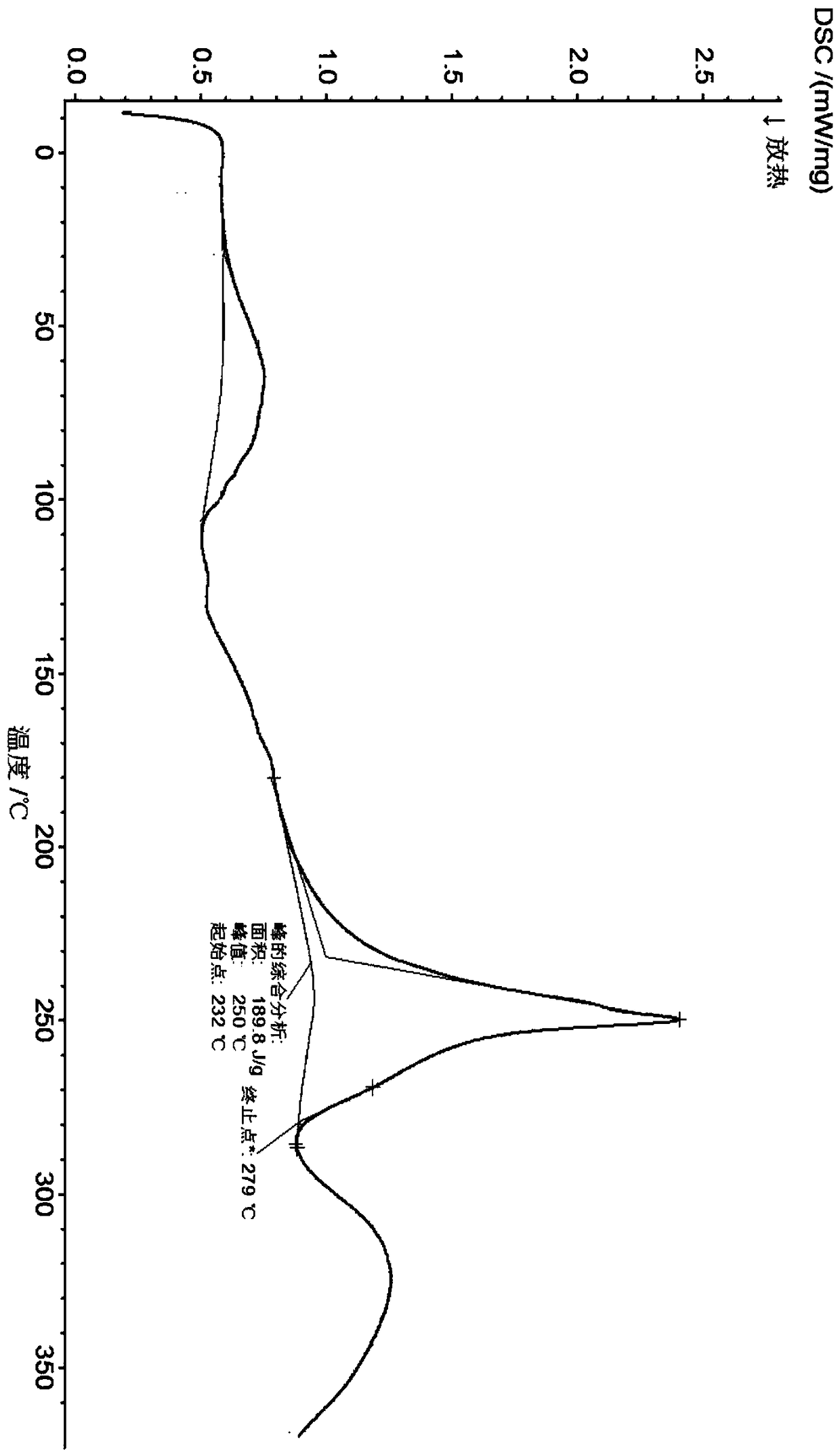
[0073] Example 3 Preparation of Compound Form H of Formula (II)
[0074] (1) In a 100mL three-necked flask, add about 7g of the pure product of structural formula I, 16.8g of methanol, stir to dissolve and then filter. Time is about 10 minutes) dropwise adding 2.8g methanolic hydrochloric acid solution (the concentration of methanolic hydrochloric acid solution is 32%-38%), no need to control the temperature, the solution is a clear solution after the dropwise addition.
[0075] (2) After the hydrochloric acid methanol solution is added dropwise, the temperature of the solution is raised to 60-65° C. to reflux, and the reflux time is about 2 hours. A large amount of white solids are precipitated in the solution.
[0076] (3) Turn off the heating of the solution accompanied by white solid precipitation after reflux, and slowly add ethyl acetate dropwise. After the dropwise addition, first cool down and stir naturally. After reaching room temperature, cool down to 10±5°C and stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com