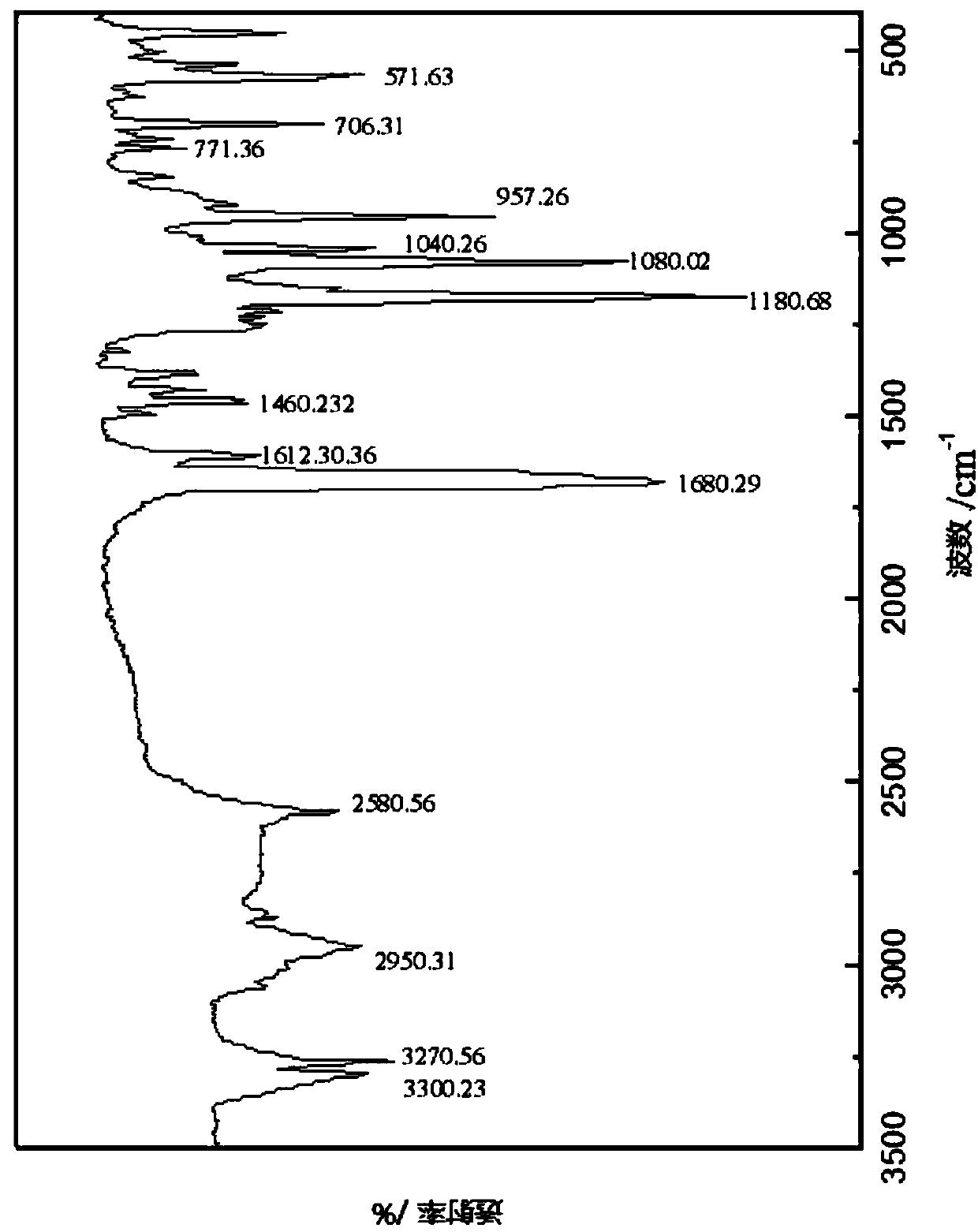
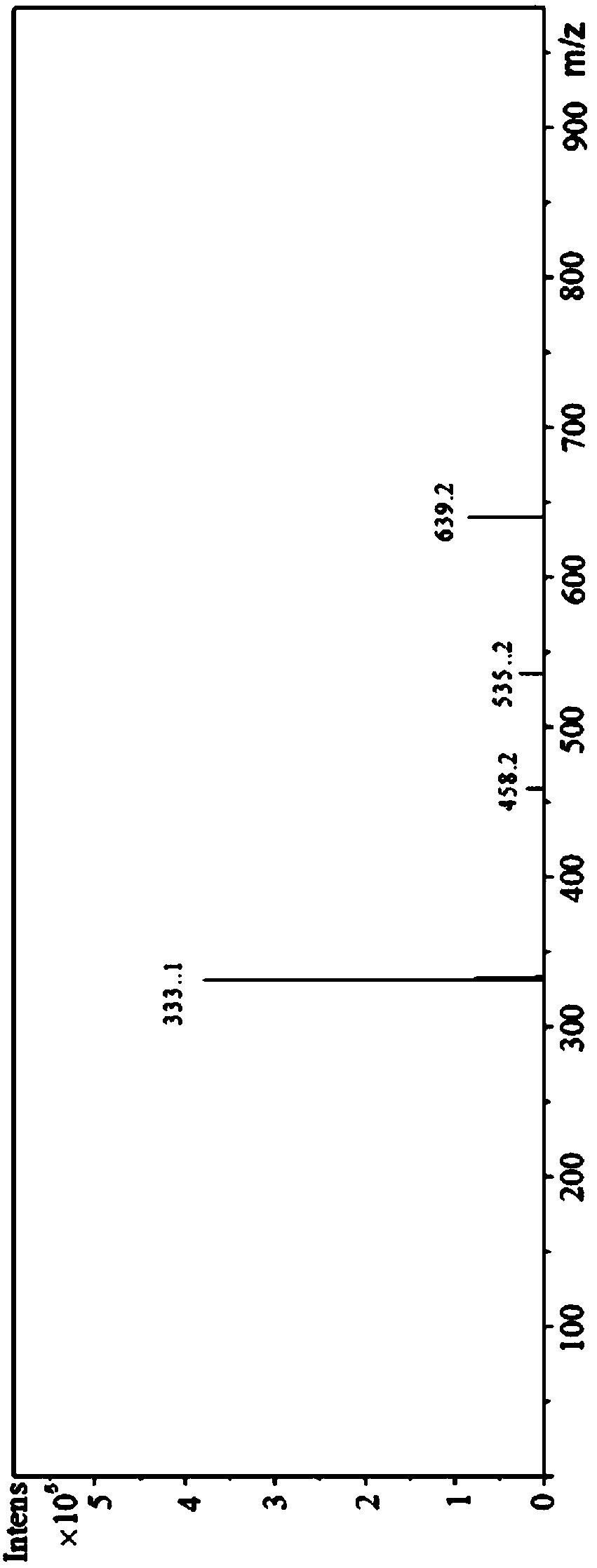
Amino phosphonic acid compound and preparation method and application thereof
An aminophosphonic acid and compound technology, which is applied in the field of aminophosphonic acid compounds, can solve the problems of complex preparation process, low synthesis yield and insignificant effect, etc., and achieves easy industrial production, high product yield and good water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, an aminophosphonic acid compound, the general formula of the compound is shown in (I):
[0023]
[0024] Wherein: R is H, F, Cl or Br.
[0025] Its preparation method includes the following steps: add aniline or aniline containing substituents, phosphorous acid solution and an acidic catalyst in a reaction kettle, raise the temperature of the reaction system to 80°C, and slowly add 3,7-dimethyl Base-2,6-octadienal, after the dropwise addition, raise the temperature of the reaction system to 110°C, react at this temperature, and obtain a white solid after cooling. The reaction time was 1 hour. The acidic catalyst used in the reaction is selected from one of hydrochloric acid, sulfuric acid, nitric acid, hydrofluoric acid or phosphoric acid; the reaction raw material is 3,7-dimethyl-2,6-octadienal, aniline or aniline containing substituents The molar ratio of phosphorous acid and acidic catalyst is 1:1:1:0.02. Aminophosphonic acid compounds are used as...
Embodiment 2
[0026] Embodiment 2, an aminophosphonic acid compound, the general formula of the compound is shown in (I):
[0027]
[0028] Where: R is C 1~6 Hydrocarbyl.
[0029] Its preparation method comprises the following steps: adding aniline or aniline containing substituents, phosphorous acid solution and an acidic catalyst into a reaction kettle, raising the temperature of the reaction system to 100°C, and slowly adding 3,7-dimethyl Base-2,6-octadienal, after the dropwise addition, raise the temperature of the reaction system to 160°C, react at this temperature, and obtain a white solid after cooling. The reaction time was 4 hours. The acid catalyst used in the reaction is selected from oxalic acid, citric acid, tartaric acid, glycolic acid, phthalic acid, sulfamic acid or p-toluenesulfonic acid. The molar ratio of the reaction raw materials 3,7-dimethyl-2,6-octadienal, aniline or aniline containing substituents, phosphorous acid and acidic catalyst is 1: 2: 3: 0.08. Aminoph...
Embodiment 3
[0030] Embodiment 3, an aminophosphonic acid compound, the general formula of the compound is shown in (I):
[0031]
[0032] Where: R is H.
[0033] Its preparation method includes the following steps: add aniline or aniline containing substituents, phosphorous acid solution and an acidic catalyst in a reaction kettle, raise the temperature of the reaction system to 90°C, and slowly add 3,7-dimethyl Base-2,6-octadienal, after the dropwise addition, raise the temperature of the reaction system to 130°C, react at this temperature, and obtain a white solid after cooling. The reaction time was 1.5 hours. The acid catalyst used in the reaction is selected from oxalic acid, citric acid, tartaric acid, glycolic acid, phthalic acid, sulfamic acid or p-toluenesulfonic acid. The molar ratio of the reaction raw materials 3,7-dimethyl-2,6-octadienal, aniline or aniline containing substituents, phosphorous acid and acidic catalyst is 1:1:1:0.05. Aminophosphonic acid compounds are us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com