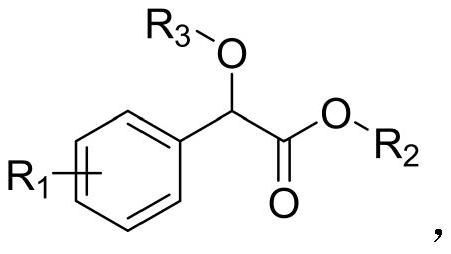
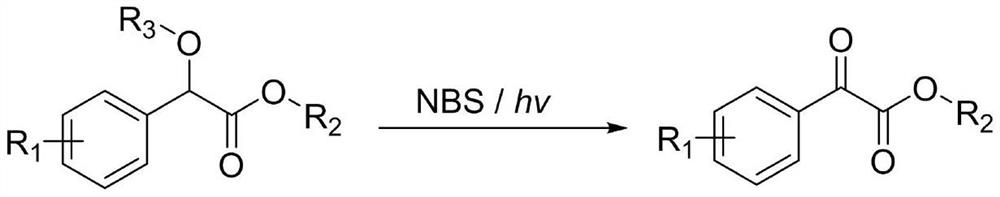
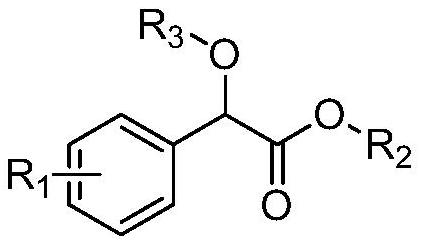
A kind of method for preparing benzoyl formate derivative
A technology of benzoyl formate and derivatives, which is applied in the field of preparation of benzoyl formate derivatives, can solve problems such as improper operation, low product yield, complicated operation, etc., and achieve mild conditions and good yield , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of embodiment 1 methyl benzoylformate
[0033] 54 mg (0.3 mmol) of alpha-methoxymethyl phenylacetate, 64 mg (0.36 mmol) of N-bromosuccinimide (NBS), were dissolved in 1 mL of ethyl acetate, and reacted for 1 hour under light conditions. After the reaction was completed, it was extracted three times with ethyl acetate, and the organic phases were combined and concentrated under reduced pressure. The crude product was separated and purified by silica gel column chromatography (diethyl ether: n-hexane = 1:50) to obtain 42 mg of a colorless liquid with a yield of 86%.
[0034] Under the same conditions, using alpha-hydroxyphenylacetic acid methyl ester as a raw material, the reaction yield of methyl benzoylformate was 80%.
[0035] Methyl benzoylformate:
[0036] 1 H NMR (400MHz, CDCl 3 )δ: 8.01 (dd, J=8.3, 1.1 Hz, 2H), 7.69–7.62 (m, 1H), 7.50 (t, J=7.8Hz, 2H), 3.97 (s, 3H).
[0037] 13 C NMR (100MHz, CDCl 3 )δ: 186.02, 164.02, 134.94, 132.42...
Embodiment 2
[0038] The preparation method of embodiment 2 m-chlorobenzoylformic acid methyl esters
[0039] 59.6 mg (0.3 mmol) of methyl 2-(3-chlorophenyl)-2-methoxyacetate, N-bromosuccinimide (NBS) (0.36 mmol), dissolved in 1 mL of ethyl acetate, in Under light conditions, react for 3 hours. After the reaction was completed, it was extracted three times with ethyl acetate, and the organic phases were combined and concentrated under reduced pressure. The crude product was separated and purified by silica gel column chromatography (diethyl ether: n-hexane = 1:50) to obtain 48 mg of white solid with a yield of 81%. Methyl m-chlorobenzoylformate:
[0040] 1 H NMR (400MHz, CDCl 3 )δ: 4.00(s, 3H), 7.47(t, J=7.9Hz, 1H), 7.64(dd, J=2.27, 7.9Hz, 1H), 7.93(d, J=8.1Hz, 1H), 8.03( s, 1H).
[0041] 13 C NMR (100MHz, CDCl 3 )δ: 53.0, 128.2, 129.8, 130.2, 133.9, 134.8, 135.2, 163.1, 184.4.
Embodiment 3
[0042] The preparation method of embodiment 3 p-chlorobenzoylformic acid methyl esters
[0043] 59.6 mg (0.3 mmol) of 2-(4-chlorophenyl)-2-methoxyacetic acid methyl ester, N-bromosuccinimide (NBS) (0.36 mmol), dissolved in 1 mL of ethyl acetate, in Under light conditions, react for 2 hours. After the reaction was completed, it was extracted three times with ethyl acetate, and the organic phases were combined and concentrated under reduced pressure. The crude product was separated and purified by silica gel column chromatography (diethyl ether: n-hexane = 1:50) to obtain 52 mg of a white solid with a yield of 87%.
[0044] Under the same conditions, using methyl 2-(4-chlorophenyl)-2-hydroxyacetate as a raw material, the yield of methyl benzoylformate was 90%.
[0045] Methyl p-chlorobenzoylformate:
[0046] 1 H NMR (400MHz, CDCl 3 )δ: 3.99 (s, 3H), 7.50 (d, J=8.5Hz, 2H), 8.00 (d, J=8.5Hz, 2H).
[0047] 13 C NMR (100MHz, CDCl 3 )δ: 52.9, 129.4, 130.8, 131.4, 141.7, 163...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com