Preparation method for ciprofloxacin sodium chloride injection
A technology for ciprofloxacin lactate and sodium chloride injection, which is applied to medical preparations containing active ingredients, the digestive system, and pharmaceutical formulas, and can solve problems such as low medicinal value, potential safety hazards, and unqualified foreign bodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
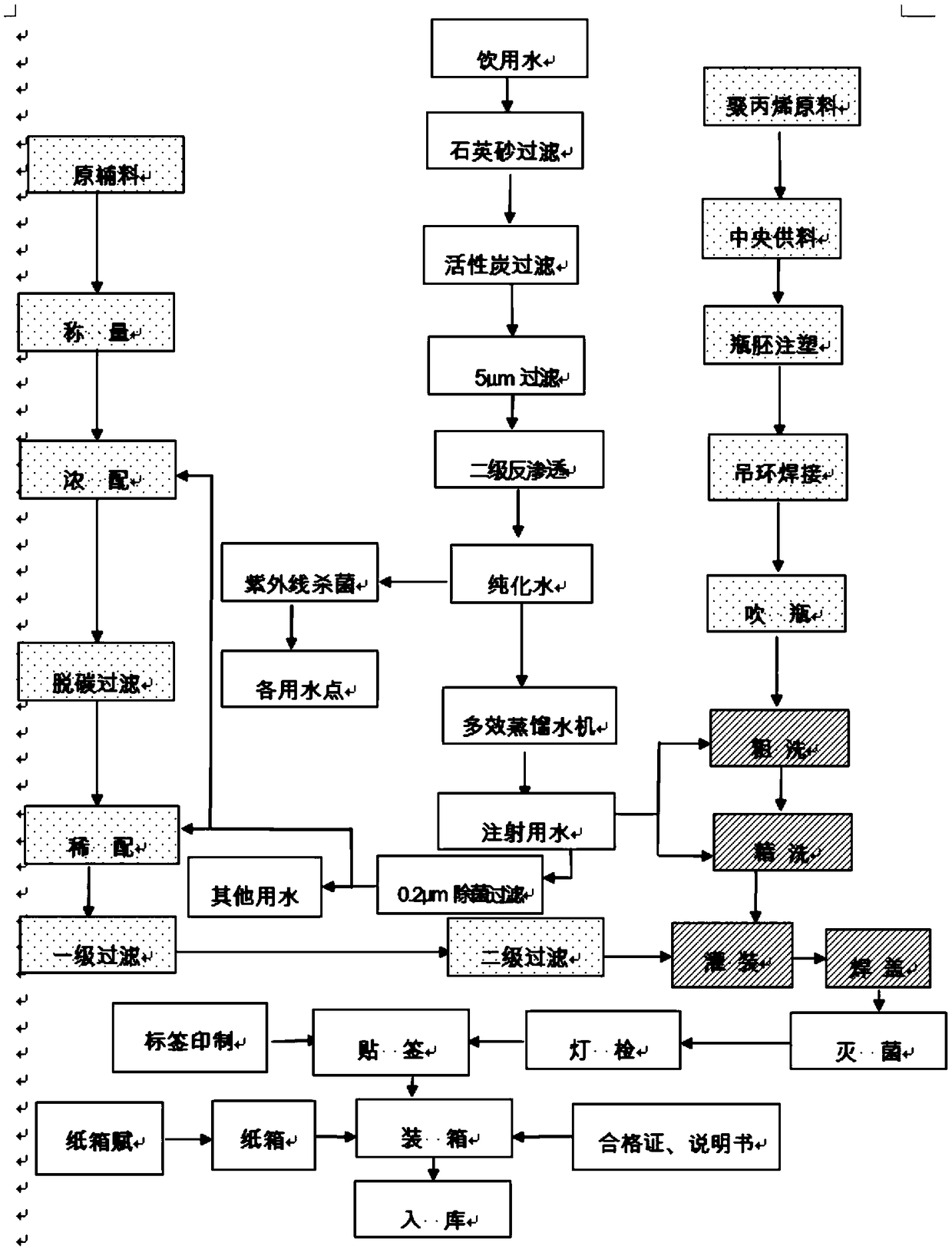
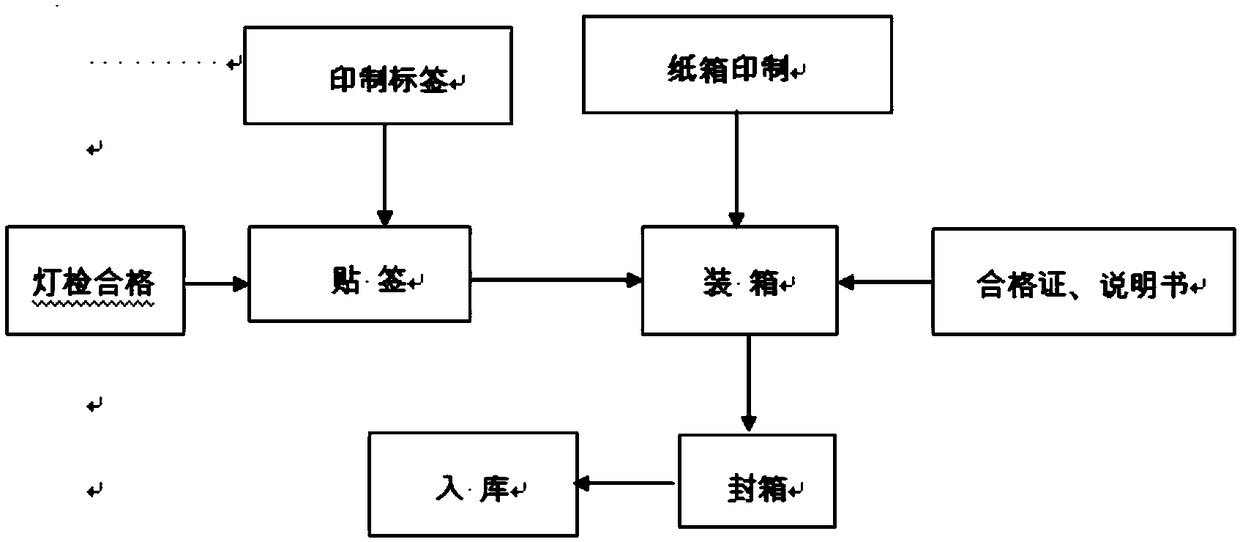
[0031] The present embodiment provides a kind of preparation method of ciprofloxacin lactate sodium chloride injection, comprising the following steps:
[0032] a. Preparation of water for injection: take drinking water, filter through quartz sand and activated carbon, pass through a 0.5 μm sieve, and the water under the sieve is processed by a reverse osmosis device to obtain purified water; At ℃, the conductivity is 3.8μs / cm, the total organic carbon is 0.38mg / L, and the flow rate is 1.89m / s;
[0033] Purified water is taken, distilled through a multi-effect distilled water machine, and then filtered through a 0.2 μm sterilizing filter to obtain water for injection, which is used as a solvent or other water; the pH value of the water for injection is 5, and at a temperature of 25°C, The conductivity is 0.6μs / cm, the total organic carbon is 0.42mg / L, the flow rate is 2.04m / s, and placed in a constant temperature circulation at a temperature of 75°C for standby; the circulatio...
Embodiment 2
[0050] The present embodiment provides a kind of preparation method of ciprofloxacin lactate sodium chloride injection, comprising the following steps:
[0051] a. Preparation of water for injection: take drinking water, filter through quartz sand and activated carbon, pass through a 0.5 μm sieve, and the water under the sieve is treated with a reverse osmosis device to obtain purified water; At ℃, the conductivity is 4.03μs / cm, the total organic carbon is 0.28mg / L, and the flow rate is 1.69m / s;
[0052] Take part of the purified water, sterilize it with ultraviolet light for 35 minutes, and then filter it through a 0.2 μm sterilizing filter to obtain water for each water point, such as washing water, soaking water, etc.;
[0053] Then take part of the purified water, carry out distillation treatment through a multi-effect distilled water machine, and then filter through a 0.2 μm sterilizing filter to obtain water for injection, which can be used as a solvent or other water; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com