Preparation method of ciprofloxacin lactate sodium chloride injection
A technology of sodium chloride injection and ciprofloxacin lactate, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve problems such as blood calcium reduction and adverse reactions, Achieve the effect of simple formula, good stability and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
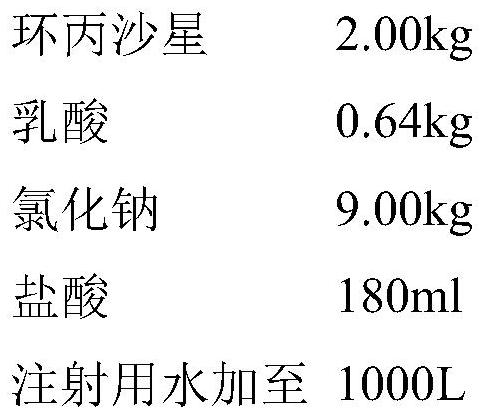
[0029] Embodiment 1 Ciprofloxacin lactate and sodium chloride injection and its preparation example
[0030] prescription:
[0031]
[0032] Preparation Process:
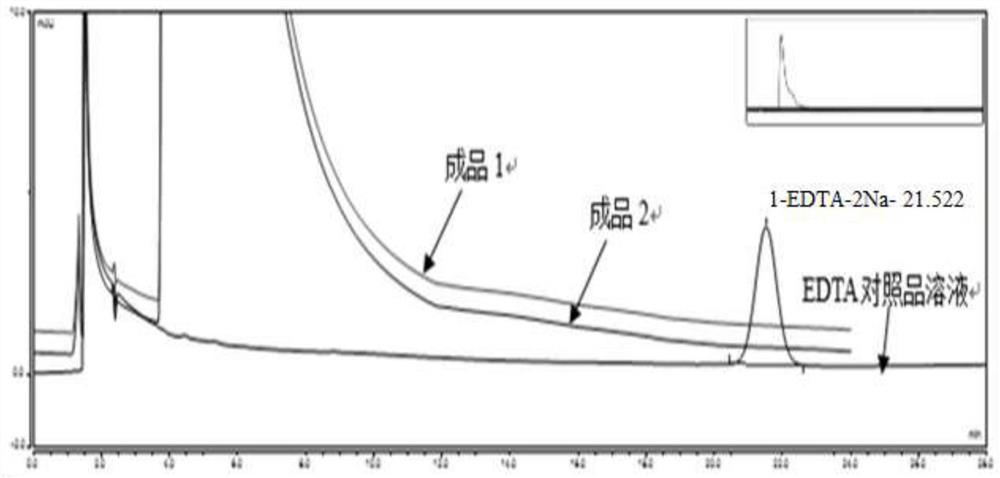
[0033] Step a, prepare 0.05% disodium edetate solution, soak the liquid dispensing tank for 40 minutes; then open the feeding valve, discharge the disodium edetate solution to the liquid delivery pipeline, clean the pipeline for 30 minutes, and then use Rinse the pipeline and dosing tank with water for injection until the flushing solution does not contain disodium edetate;
[0034] In step b, lactic acid and hydrochloric acid are added to 5L of water for injection to prepare a solution, and the solution is heat-treated at 121°C for 30 minutes;
[0035] Step c, disperse ciprofloxacin in the water for injection in the dosing tank processed in step a, add the lactic acid solution of step b, stir to dissolve ciprofloxacin;
[0036] Step d, adding sodium chloride to the solution of step c, stirring and dissolving;...
Embodiment 2
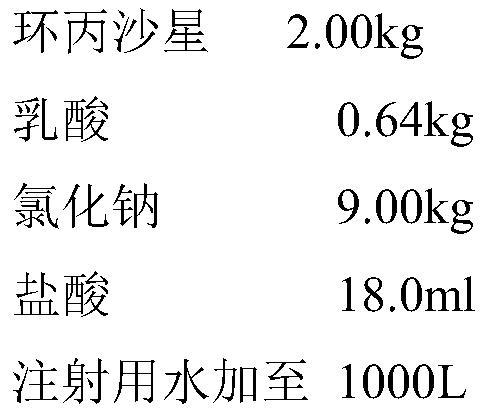
[0043] Embodiment 2 Ciprofloxacin lactate and sodium chloride injection and its preparation example
[0044] prescription:
[0045]
[0046] Preparation Process:
[0047] Step a, prepare 0.02% disodium edetate solution, soak the liquid dispensing tank for 60 minutes; then open the feeding valve, discharge the disodium edetate solution to the liquid delivery pipeline, clean the pipeline for 30 minutes, and then use Rinse the pipeline and dosing tank with water for injection until the flushing solution does not contain disodium edetate;
[0048] In step b, lactic acid and hydrochloric acid are added to 5L of water for injection to prepare a solution, which is heat-treated at 115°C for 45 minutes;
[0049] Step c, ciprofloxacin is dispersed in the water for injection in the dosing tank processed by step a, the lactic acid solution of step b is added, and stirring makes ciprofloxacin dissolve;
[0050] Step d, adding sodium chloride to the solution of step c, stirring and diss...
Embodiment 3
[0057] Embodiment 3 product quality and stability research
[0058] The Ciprofloxacin Lactate Sodium Chloride Injection prepared by Example 1 and Example 2 was placed under normal temperature conditions (product storage conditions) for 24 months, and in 0, 3, 6, 9, 12, 18, 24 months Sampling, according to the 2020 version of the Chinese Pharmacopoeia standard to test each quality index of the injection, the results are shown in Table 1 and Table 2 below.
[0059] Table 1 Example 1 finished product and stability results
[0060]
[0061] Table 2 Example 2 finished product and stability results
[0062]
[0063] The results show that the ciprofloxacin lactate sodium chloride injection of the present invention is stored for 24 months under the product storage conditions, and the quality is stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com