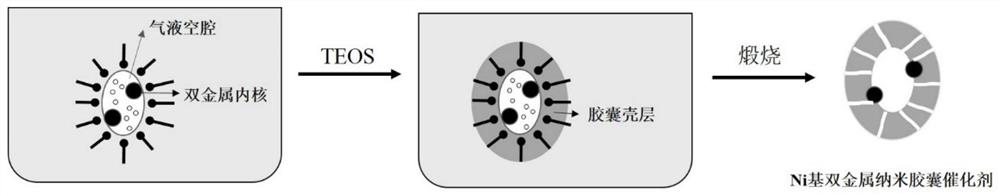
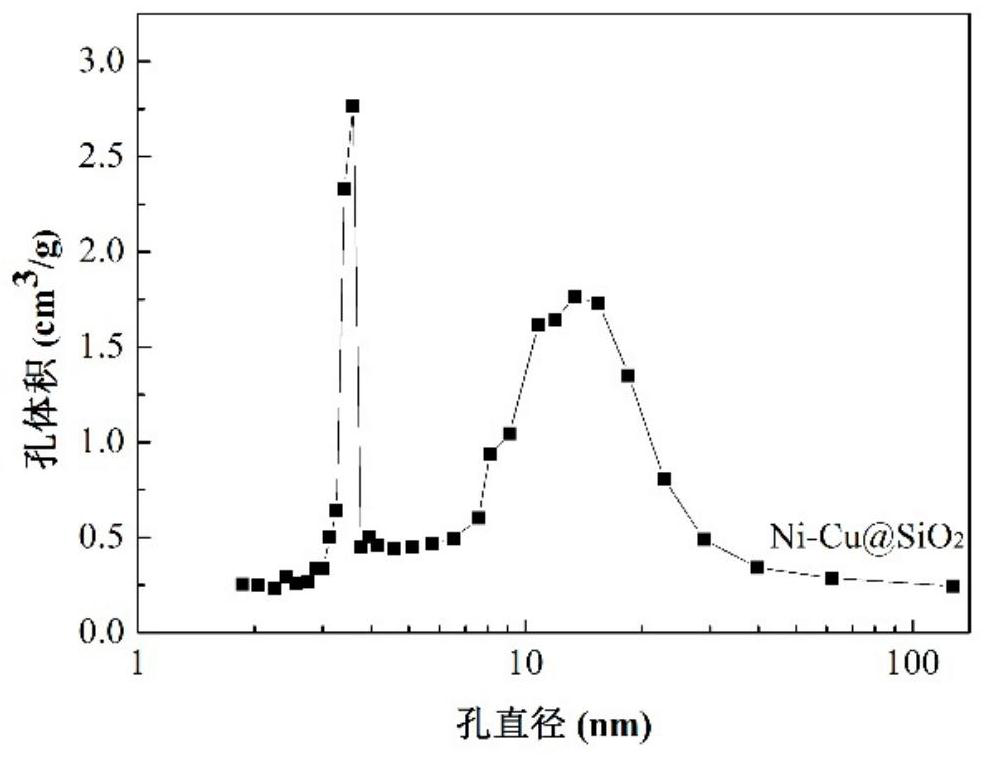
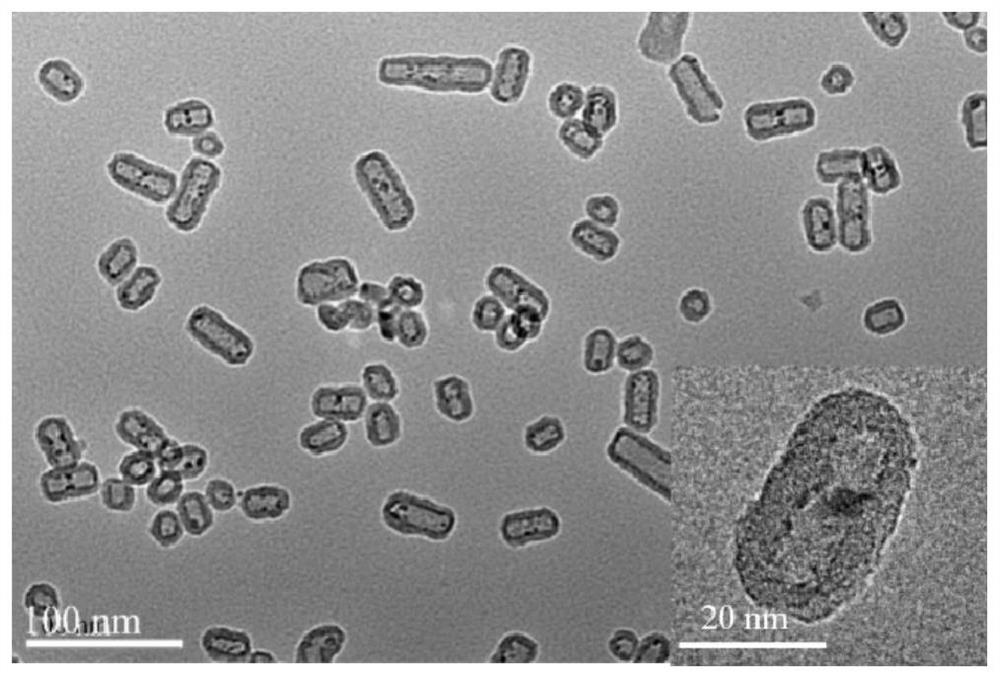
A kind of Ni-based bimetallic nanocapsule catalyst and its preparation and application
A technology of nanocapsules and catalysts, which is applied in Ni-based bimetallic nanocapsule catalysts and its preparation, and in the application fields of biomass gas reforming reactions. Effect and other issues, to achieve the effect of reducing the potential of the reduction electrode, promoting rapid formation, and avoiding mutual interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Weigh 10 g of polyoxyethylene (10) cetyl ether into a conical flask, add cyclohexane to 100 mL, heat and stir at 40° C. When it is observed that the solution becomes clear, add 5 mL of 2.0 mol / L nickel nitrate and copper nitrate mixed solution (Ni:Cu molar ratio is 1:1), stir until well mixed, then add 2 mL of hydrazine hydrate. After aging for 0.5h, increase the rotation speed and add a mixed solution of 2ml of concentrated ammonia water (25wt.%) and 13mL of deionized water, then slowly add 10mL of TEOS, after hydrolysis for 1h, add isopropanol to break the emulsion, and centrifuge. Finally, the obtained sample was dried at 110° C. for 12 h, and then calcined at 500° C. at a rate of 1° C. / min in an air atmosphere to obtain bimetallic nanocapsule catalyst 1 .
[0036] The calcined catalyst 1 was pressed into tablets and sieved, and 0.1g of the 20-40 mesh catalyst was taken, mixed evenly with quartz sand and then put into a reaction tube, and then reduced for ...
Embodiment 2
[0037] Example 2: Weigh 20 g of polyoxyethylene (10) cetyl ether into a conical flask, add cyclohexane to 100 mL, heat and stir at 45° C. When it is observed that the solution becomes clear, add 7 mL of 1.8 mol / L nickel nitrate and cobalt nitrate mixed solution (Ni:Co molar ratio is 2:1), stir until well mixed, then add 3 mL of hydrazine hydrate. After aging for 1.5 hours, increase the speed and add a mixed solution of 1.5ml of concentrated ammonia water and 13.5mL of deionized water, then slowly add 12.5mL of TEOS, after hydrolysis for 6 hours, add isopropanol to break the emulsion, and centrifuge. Finally, the obtained sample was dried at 80° C. for 24 h, and then calcined at 600° C. at a rate of 1.5° C. / min in an air atmosphere to obtain bimetallic nanocapsule catalyst 2 .
[0038] The calcined catalyst 2 was pressed into tablets and sieved, and 0.1g of 40-60-mesh catalyst was taken, mixed evenly with quartz sand, and then put into a reaction tube, and then reduced for 1 ho...
Embodiment 3
[0039] Example 3: Weigh 34g of polyoxyethylene (10) cetyl ether into a conical flask, add cyclohexane to 100mL, heat and stir at 50°C. When it is observed that the solution becomes clear, add 5 mL of 1.5 mol / L nickel nitrate and copper nitrate mixed solution (Ni:Cu molar ratio is 4:1), stir until well mixed, then add 4 mL of hydrazine hydrate. After aging for 3 hours, increase the rotation speed and add a mixed solution of 1ml of concentrated ammonia water and 14mL of deionized water, then slowly add 10mL of TEOS, after hydrolysis for 12h, add isopropanol to break the emulsion, and centrifuge. Finally, the obtained sample was dried at 100° C. for 12 h, and then calcined at 700° C. at a rate of 2° C. / min in an air atmosphere to obtain bimetallic nanocapsule catalyst 3 .
[0040] The calcined catalyst 3 was pressed into tablets and sieved, and 0.1g of 20-40 mesh catalyst was taken, mixed evenly with quartz sand and then put into a reaction tube, and then reduced for 1 hour at 70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com