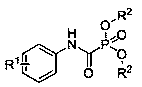
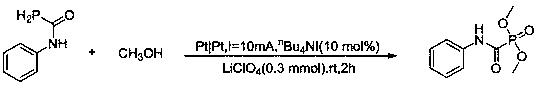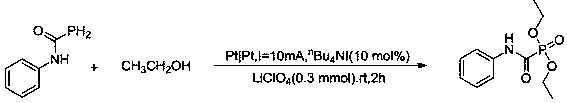Method for preparing N-aryl amido formyl phosphonate through anode oxidization
An aryl carbamoyl phosphonate, anodic oxidation technology, applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of inability to realize the reaction, meet the requirements of avoiding use, mild reaction conditions, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the preparation of dimethyl (p-tolylcarbamoyl) phosphonate
[0021] In a three-necked flask, add 10 mL of methanol, 0.3 mmol of lithium perchlorate, 10 mol% of tetrabutylammonium iodide, and 0.2 mmol of N-phenylphosphoric carboxamide in sequence. Stir at room temperature, and use a platinum sheet as an electrode. Electrolyze at a constant current for about 2 h at I = 10 mA (until the reaction solution changes from colorless to light yellow); after the reaction is detected by TCL, add 2 mL of saturated ammonium chloride to quench the reaction, and use an excess (3×15 mL) of ethyl acetate was extracted quickly, dried over anhydrous magnesium sulfate, and separated by column chromatography (eluent was ethyl acetate:petroleum ether=1:2) to obtain the pure product——dimethyl (p-tolyl carbamoyl) phosphonate. Yield 92%. Its synthetic formula is as follows:
[0022]
[0023] 1 H NMR (600 MHz, CDCl 3 ) δ=8.87 (s, 1H), 7.63 (d, J = 8.4Hz, 2H), 7.35(t, J =...
Embodiment 2
[0024] Embodiment 2: the preparation of diethyl ((4-methoxyphenyl) carbamoyl) phosphonate
[0025] In a three-necked flask, add 10 mL ethanol, 0.3 mmol lithium perchlorate, 10 mol% tetrabutylammonium iodide, 0.2 mmol N-(4-methoxyphenyl)phosphorocarboxamide, and stir at room temperature Then, use platinum sheet as electrode, and conduct constant current electrolysis at I = 10 mA for about 2 h (until the reaction solution changes from colorless to light yellow); after the reaction is detected by TCL, 2 mL of saturated ammonium chloride is added to quench the reaction. The reaction was quickly extracted with excess (3×15 mL) ethyl acetate, dried over anhydrous magnesium sulfate, and separated by column chromatography (eluent: ethyl acetate:petroleum ether=1:2) to obtain the pure product— - Diethyl((4-methoxyphenyl)carbamoyl)phosphonate. Yield 85%. Its synthetic formula is as follows:
[0026]
[0027] 1 H NMR (600 MHz, CDCl 3 ) δ=8.90 (s, 1H), 7.55 (d, J = 1.2Hz, 2H), 6...
Embodiment 3
[0028] Embodiment 3: Preparation of diisopropyl (o-tolylcarbamoyl) phosphonate
[0029] In a three-necked flask, 10 mL of isopropanol, 0.3 mmol of lithium perchlorate, 10 mol% of tetrabutylammonium iodide, 0.2 mmol of N-(2-methyl-phenyl)phosphorocarboxamide were added successively, and Stir at room temperature, use a platinum sheet as an electrode, and conduct constant current electrolysis at I = 10mA for about 2 h (until the reaction solution changes from colorless to light yellow); after the reaction is detected by TCL, add 2 mL of saturated ammonium chloride to quench the reaction. Reaction, rapid extraction with excess (3×15mL) ethyl acetate, drying over anhydrous magnesium sulfate, separation by column chromatography (eluent: ethyl acetate:petroleum ether=1:2), to obtain the pure product—— Diisopropyl(o-tolylcarbamoyl)phosphonate. The yield is 80%. Its synthetic formula is as follows:
[0030]
[0031] 1 H NMR (600 MHz, CDCl 3 ) δ= 8.64 (s, 1H), 7.94 (d, J = 7.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com