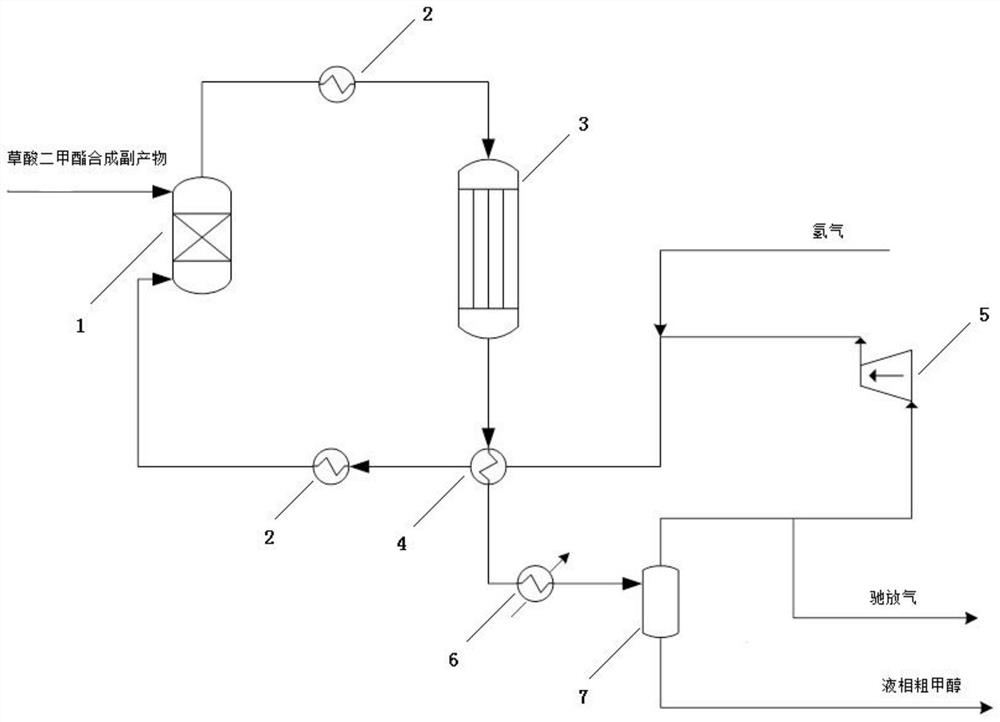
A recovery method and recovery system for by-products in a dimethyl oxalate synthesis process
A technology of dimethyl oxalate and synthesis process, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of complex recovery process, high number of material cycles, and high recovery costs, and achieve efficient and clean recovery. The effect of the reaction process, reducing equipment investment and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The mixed by-products collected by the recovery tower are mixed with the circulating hydrogen, and the circulating hydrogen volume is 30600 Nm 3 / h, the reaction pressure is 2.80 MPa, the reaction hydrogen-ester ratio is 60, and the reaction temperature is 200°C. The conversion rates of dimethyl carbonate and methyl formate were 99.8% and 99.9%, respectively. Methanol selectivity 100%, space-time yield STY: 1079 Kg / M 3 cata.h.
Embodiment 2
[0048] The mixed by-products collected by the recovery tower are mixed with the circulating hydrogen, and the circulating hydrogen volume is 30600 Nm 3 / h, the reaction pressure is 2.0 MPa, the reaction hydrogen-ester ratio is 60, and the reaction temperature is 160°C. The conversions of dimethyl carbonate and methyl formate were 95.1 and 96.2 %, respectively. Methanol selectivity 100%, space-time yield STY: 1029 Kg / M 3 cata.h.
Embodiment 3
[0050] The mixed by-products collected by the recovery tower are mixed with the circulating hydrogen, and the circulating hydrogen volume is 30600 Nm 3 / h, the reaction pressure is 2.0 MPa, the reaction hydrogen-ester ratio is 60, and the reaction temperature is 240°C. The conversions of dimethyl carbonate and methyl formate were 99.9 and 99.9 %, respectively. Methanol selectivity 100%, space-time yield STY: 1080 Kg / M 3 cata.h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com