Vinyl ether Hg<2+> fluorescent probe and preparation method and application thereof
A technology of fluorescent probes and vinyl ethers, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of expensive instruments, slow detection speed, complicated operation, etc., and achieve simple steps, high sensitivity, specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Synthesis of Compound 2
[0036]Under nitrogen protection, compound 1 (2-hydroxy-1-naphthaldehyde, 500mg, 2.9mmol) and potassium carbonate (400mg, 2.9mmol) were placed in a 50mL round bottom flask, dissolved with 10mL acetone, and then added 1,2- Dibromoethane (1.3mL, 15.1mmol), the mixed solution was refluxed for 3h, and the progress of the reaction was detected by TLC thin-layer chromatography. After the raw materials were completely reacted, the reaction system was washed with 100mL of water and extracted with ethyl acetate (3×40mL). The organic phase was dried over anhydrous sodium sulfate and concentrated in vacuo. The product was purified by column chromatography to obtain 0.5 g of light yellow compound 2 (2-(2-bromoethoxy)-1-naphthaldehyde), with a yield of 63%.
[0037] 1 H NMR (400MHz, CDCl 3 )δ10.97(s,1H),9.28(d,J=8.8Hz,1H),8.07(d,J=9.2Hz,1H),7.79(d,J=8.4Hz,1H),7.64(t, J=8.4Hz, 1H), 7.45(t, J=7.6Hz, 1H), 4.56(t, J=6.0Hz, 2H), 3.74(t, J=6.0Hz, 2H...
Embodiment 2
[0045] Fluorescence Spectrometry Conditions
[0046] The width of the excitation slit is 10.0nm, the width of the emission slit is 10.0nm, the excitation wavelength is 426nm, the wavelength range is 440-700nm, the response time is 1s, the sensitivity is high, and the sample cell is a double-pass quartz cuvette with 1cm optical path.
Embodiment 3
[0048] Screening of Fluorescent Test Conditions
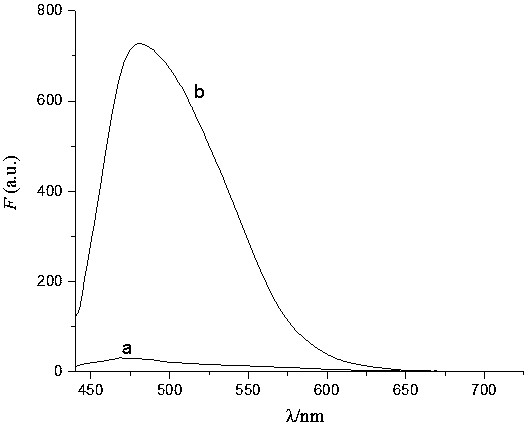
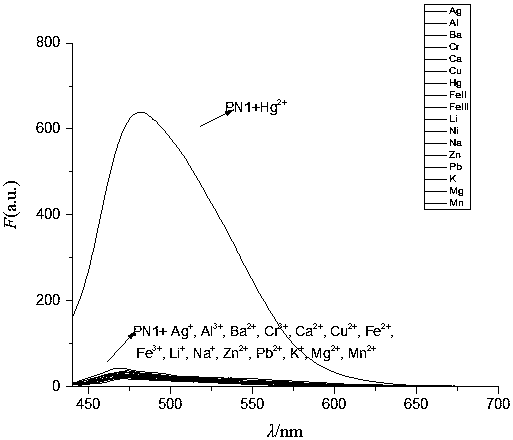
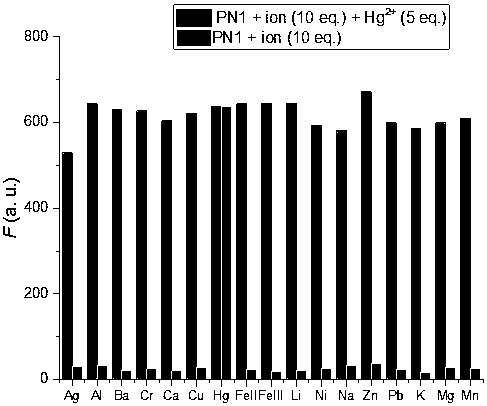
[0049] pH versus fluorescent probes PN1 and PN1+Hg 2+ Influence of Fluorescence Spectrum
[0050] Prepare buffer solutions of different systems to determine the detection of Hg by probe PN1 2+ In the optimal pH range, the fluorescence intensity of probe PN1 did not change significantly in the range of pH 2.0-12.0. While adding an equivalent amount of Hg 2+ Finally, when the pH is in the range of 6.0 to 8.0, PN1+Hg 2+ The fluorescence intensity is almost constant, and the fluorescence intensity changes with the change of pH in the range of pH8.0. Therefore, when the pH is in the range of 6.0 to 8.0, the fluorescent probe PN1 has an effect on Hg 2+ The detection is not affected by pH. Therefore, the ion selectivity, competition and concentration titration of the probes in the experiment were all carried out in PBS buffer solution with pH=7.2.
[0051] Effect of Time on Probe Stability
[0052] In the PBS buffer solution wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com