Disulfide bond containing compound and use and preparation methods thereof
A compound and disulfide bond technology, applied in the field of compounds containing disulfide bonds, can solve the problems of difficulty in separating detergents and extracted substances, and achieve the effects of good film dissolution and protein stabilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Disulfide bond bridged cleavable detergent molecule and its synthesis
[0063] 1. Disulfide bridged detergents, the structural formulas of which are the above-mentioned (XII)-(XV).
[0064] 2. The synthesis method is as follows, reagents and reaction conditions: i) sodium thiobenzenesulfonate, tetrabutylammonium bromide, acetonitrile, 70°C; ii) triethylamine, dichloromethane, 0°C to room temperature; iii ) sodium methoxide, methanol, room temperature.
[0065]
[0066] Specific steps are as follows:
[0067] (i) Dissolve 14g of alpha-D-bromomaltose heptaacetate 1 in 20ml of anhydrous acetonitrile, add 644mg of tetrabutylammonium bromide (Titan, product number: G28296B) and 8g of sodium thiobenzenesulfonate (Datang Medicine, Cat. No.: BL-06097). The reaction solution was refluxed at 70° C. for 6 hours (or reacted overnight). After the reaction was completed, the solvent was removed by rotary evaporation. Add 100mL ethyl acetate and 100mL water, separate...
Embodiment 2
[0084] Example 2 Evaluate the micelle properties formed by each detergent in Example 1
[0085] 1. CMC determination
[0086] The critical micelle concentration (CMC) of each detergent was determined by fluorescent dye method. The disulfide bond bridged detergents with structural formulas (XII)-(XV) are formulated into a gradient solution, and the CMC value of the detergent is determined according to the mutation point of the fluorescence curve of the hydrophobic fluorescent dye. For example, prepare concentrations of 0, 42, 84, 126, 168, 204, 240, 360, 480 with double distilled water containing 40uM fluorescent dye 8-amino-1-sulfonic acid naphthalene ammonium salt (Baringwei, product number: 442848). , 600, 800 and 1000uM detergent solutions and incubate for 20 minutes in the dark. Measure the fluorescence curve at 477nm / 388nm, and the abrupt change point of the curve is the CMC value of the detergent.
[0087] 2. Dynamic Light Scattering Experiment
[0088] Structural fo...
Embodiment 3
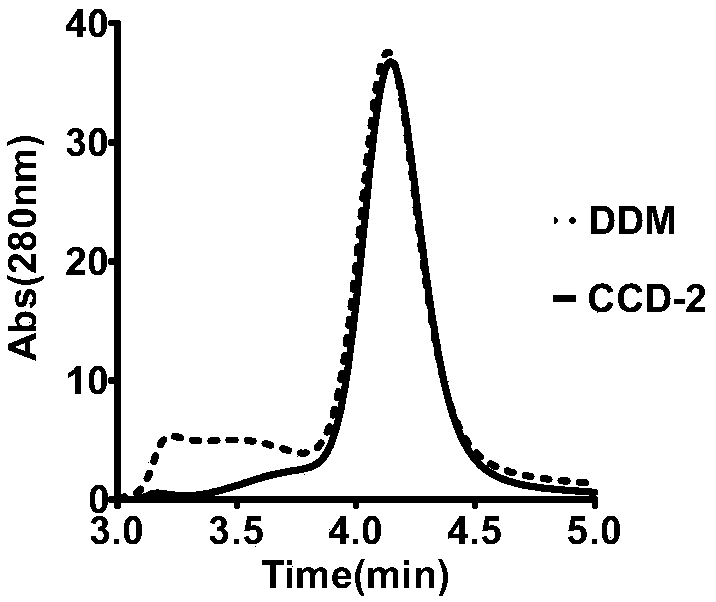
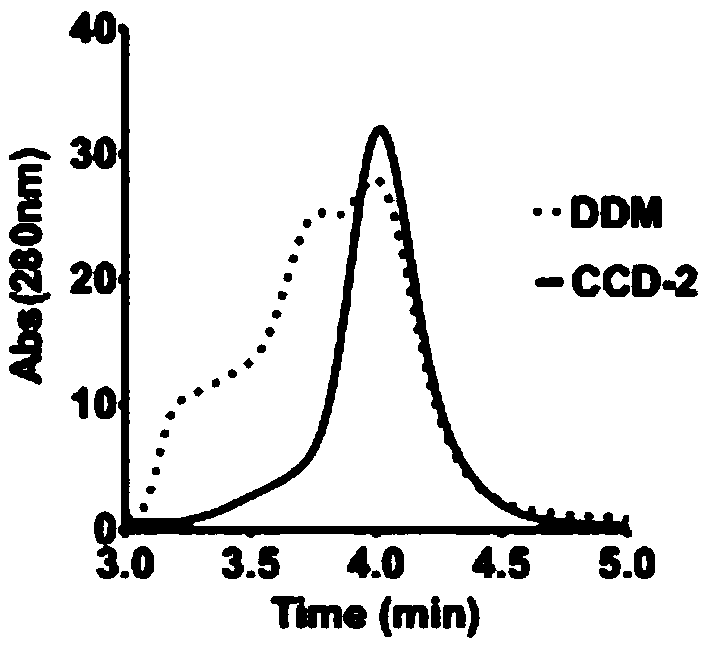
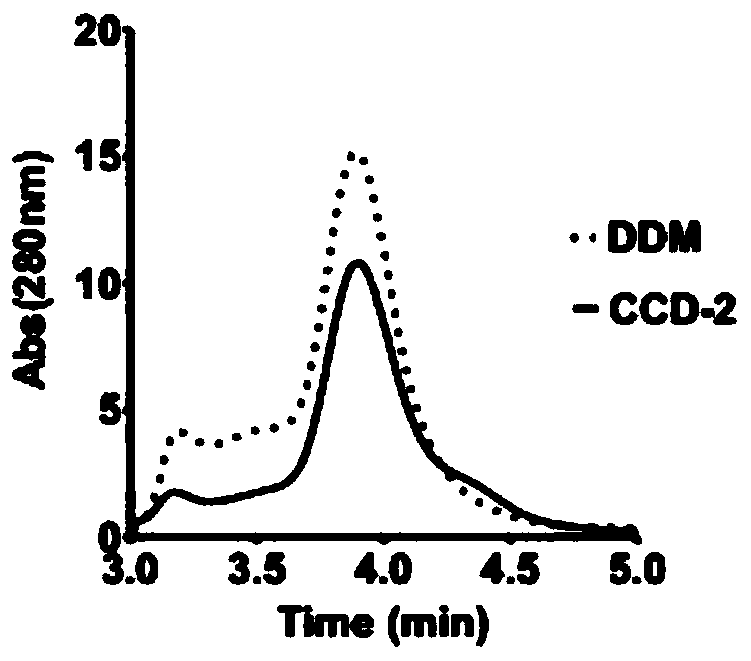
[0093] Example 3 Evaluation of CCD-2
[0094] The detergent CCD-2 in Example 1 was used to dissolve and purify GPCRs of different families, specifically including adenosine receptor 2A subtype protein (A2a protein, class A GPCR), glucagon-like peptide-1 receptor protein (GLP-1R, class B GPCR) and Smoothened receptor (SMO, class F GPCR).
[0095] 1. Specific steps:
[0096] Expression of GPCRs (A2a, GLP-1R, and SMO) in SF9 cells (invitrogen, Cat. No. 11496-015):
[0097] Cells were cultured at 37°C until the cell density reached 1.0-1.3x10 6 cells / mL were collected and broken to obtain cell membranes. Wash the cell membrane with low-salt buffer (10mM magnesium chloride (Sigma-Aldrich, product number M4880), 20mM potassium chloride (Sigma-Aldrich, P9541), 20mM Hepes (ABCONE, product number H33755) at pH 7.5) (50mL*3 times) and centrifuged, and then washed with high-salt buffer (1M sodium chloride (Shanghai Sangon, product number A610476), 10mM magnesium chloride, 20mM potass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com