Use of 2,4-thiazolidinedione compound K145 in preparation of diabetes treatment drug
A technology for thiazolidinediones and diabetes drugs, applied in the field of pharmaceutical preparations, can solve problems such as unreported effects, and achieve the effects of lowering fasting blood sugar, good hypoglycemic effect, and enhanced glucose output capacity of liver tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 2,4-thiazolidinedione compound K145 is added with pharmaceutically acceptable excipients to prepare various dosage forms according to conventional methods, such as liquid injections of various specifications, powder injections, emulsions for injection, tablets, pills, capsules, Ointments, creams, patches, liniments, powders, sprays, implants, drops, suppositories, ointments, confectionery, etc.
[0015] The route of administration includes various routes of administration: oral administration, injection administration, implant administration, intracavity administration, sublingual administration, anal administration, transdermal administration, internal and external application, etc.
[0016] In order to prove the technical means, purpose and experimental effect of the experimental invention, the following will further illustrate the present invention with specific experimental examples.
experiment example 1
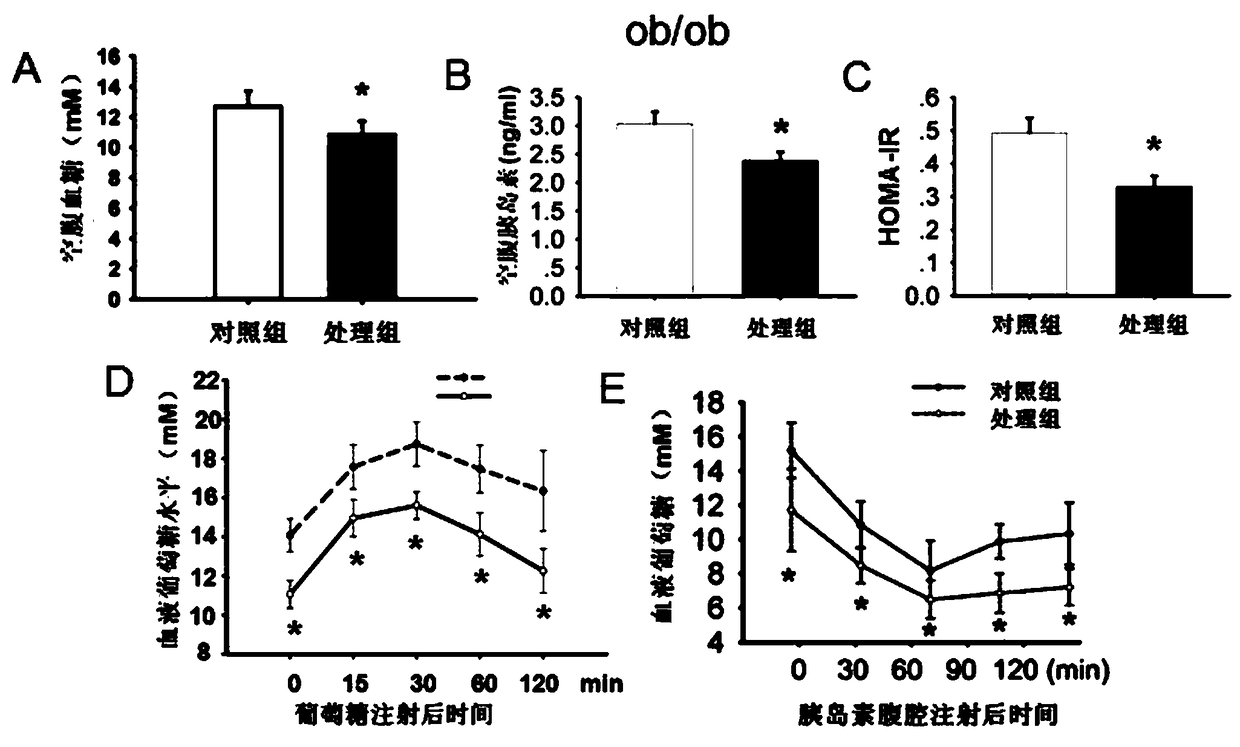
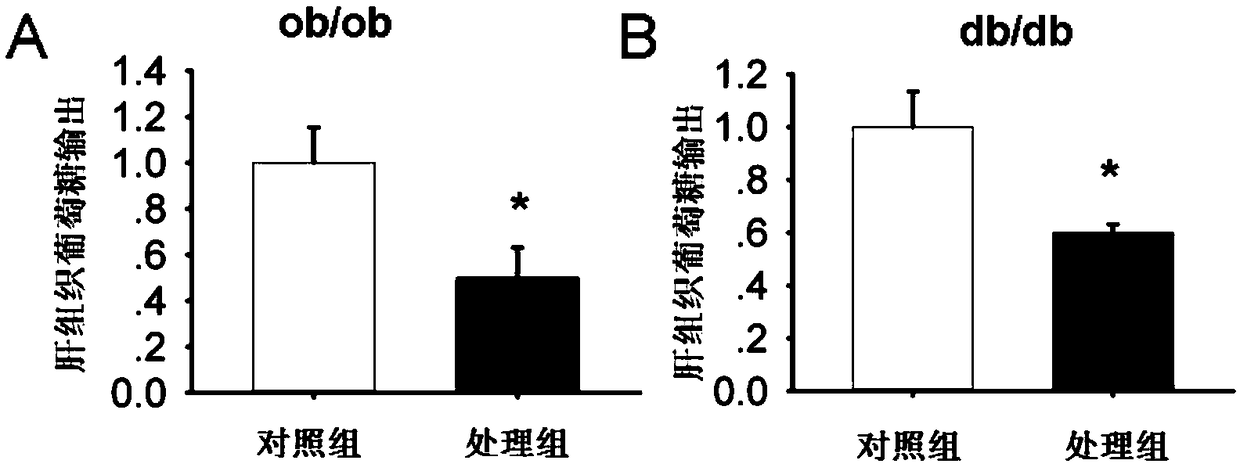
[0017] Experimental example 1: The effect of the target compound of the present invention on ob / ob diabetic mice
[0018] Animal group processing and detection indicators: 6-7 weeks old diabetic ob / ob mice, male, weighing 35-40g (provided by Nanjing Institute of Biomedicine, Nanjing University), free drinking and eating, randomly divided into DMSO intervention control group and K145 The intervention model was given by intraperitoneal injection for 17 days, once a day, with a dose of 15 mg / kg. Insulin tolerance analysis (ITT) was performed by intraperitoneal injection of 0.75IU / kg body weight of insulin; overnight starvation, intraperitoneal injection of 2g / kg body weight of aD-glucose, tail vein blood test (IPGTT); mice fasted overnight, tail removed Venous blood was used to measure fasting blood glucose and fasting insulin (RIA, Millipore, USA); according to the formula HOMA-IR = fasting blood glucose (mg / dl) X fasting insulin (mU / ml) / 405, calculate the HOMA-IR value.
[0019] Th...
experiment example 2
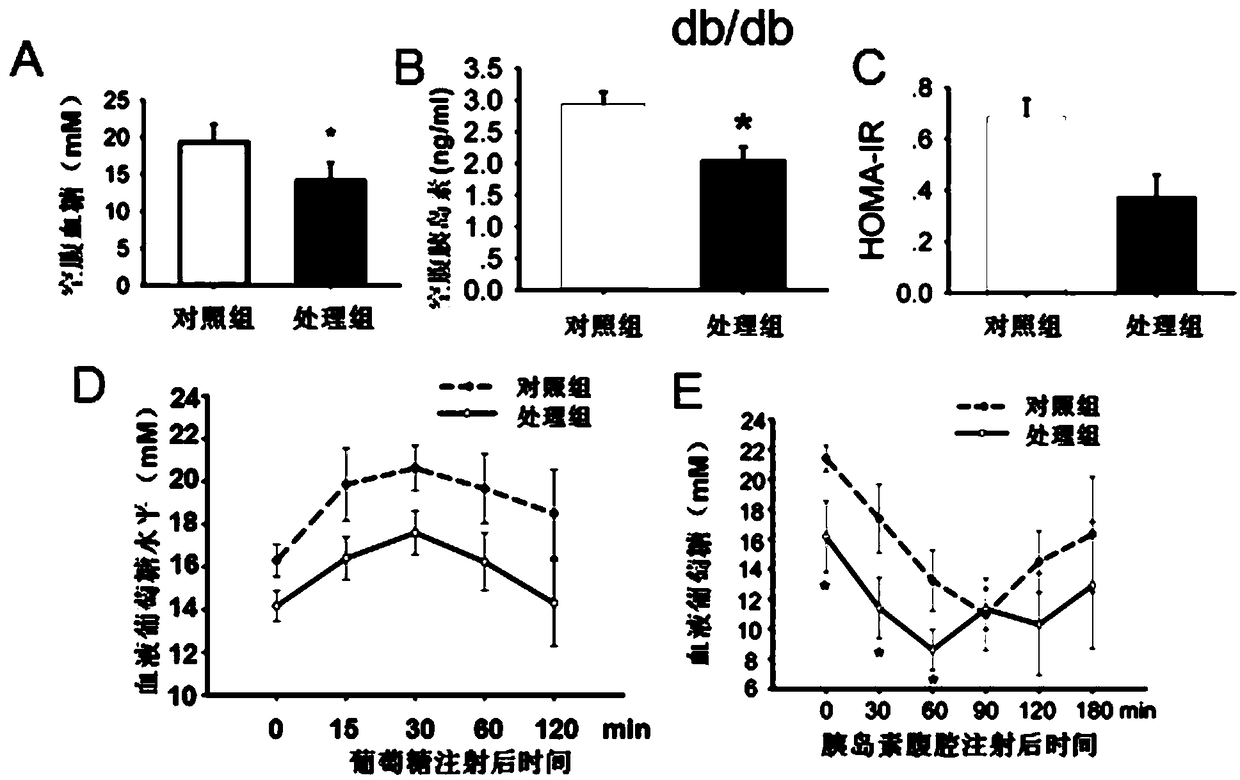
[0020] Experimental Example 2: Effect of the target compound of the present invention on db / db diabetic mice
[0021] Animal group processing and detection indicators: 6-7 weeks old diabetic db / db mice, male, weight 34-37g (provided by Nanjing Institute of Biomedicine, Nanjing University), free drinking and eating, randomly divided into DMSO intervention control group and K145 In the intervention model, the drug dissolution method is as follows: after 2% DMSO is dissolved, it is prepared into a suspension with hydroxymethyl cellulose, which is given by intragastric administration for 30 days, once a day, at a dosage of 30 mg / kg. Insulin tolerance analysis (ITT) was performed by intraperitoneal injection of 0.75IU / kg body weight of insulin; overnight starvation, intraperitoneal injection of 2g / kg body weight of aD-glucose, tail vein blood test (IPGTT); mice fasted overnight, tail removed Fasting blood glucose was measured by venous blood, and fasting insulin (RIA, Millipore, USA) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com