Triptolide targeting prodrug and its preparation method and application
A technology of triptolide and pharmaceutical preparations, applied in the field of medicine, can solve problems such as application limitations, narrow therapeutic window, and high toxicity, and achieve the effects of effective development and utilization, reduced toxicity, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
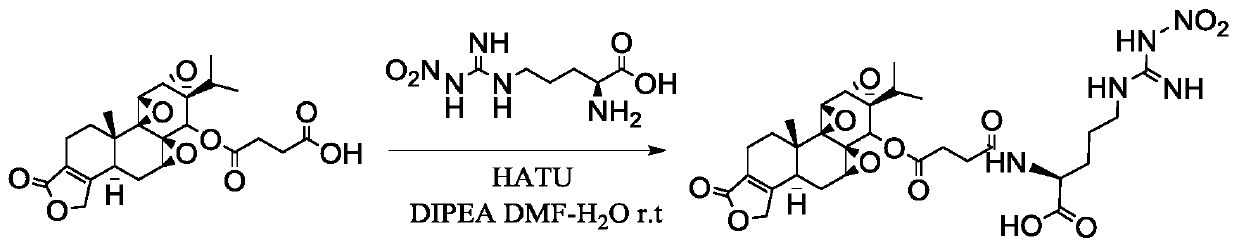
[0033] The specific steps of the preparation method of the triptolide targeted prodrug provided by the invention are:
[0034] 1. Activation of the terminal carboxyl group of triptolide succinate (active ester intermediate)
[0035] Use THF-water (v:v) = 10:1.5 as solvent, stirring and dissolving triptolide succinate with 1 part by weight, adding triethylamine with 2.02-4.06 parts by weight and stirring evenly, batchwise slowly Add 0.52-2.29 parts by weight of the activator, stir and activate at room temperature for 45min-1h, after TLC detects that the activation is complete, cool to -5°C to -30°C in a low-temperature reactor, and stir for later use.
[0036] 2. Active ester intermediate amidation stage
[0037] In addition, weigh 1.23 to 2.36 parts by weight of amino acid, dissolve it in 500 μL of water, adjust the pH of the aqueous solution of amino acid to the isoelectric point of the amino acid, and use a micropipette to add the aqueous solution of amino acid dropwise in ...
Embodiment 1
[0040] The preparation of embodiment 1 triptolide succinate L-arginine amide
[0041] The preparation method of triptolide targeting prodrug comprises the following steps:
[0042] A 1 : Activation of triptolide succinate terminal carboxyl group
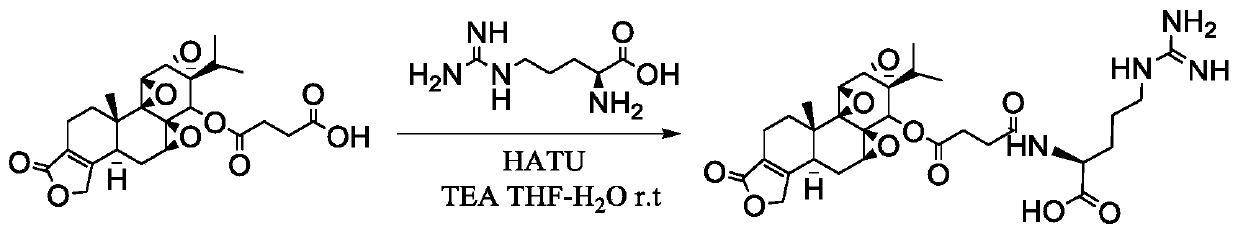
[0043] Such as figure 1The preparation process shown. Use THF-water (v:v) = 10:1.5 as solvent, stirring and dissolving triptolide succinate with 1 part by weight, adding triethylamine with 2.02-4.06 parts by weight and stirring evenly, batchwise slowly Add 0.52-2.29 parts by weight of the activator, stir and activate at room temperature for 45min-1h, after TLC detects that the activation is complete, cool to -5°C to -30°C in a low-temperature reactor, and stir for later use.
[0044] B 1 : Active ester intermediate amidation stage
[0045] Another 25 mg (0.141 mmol) of L-arginine was weighed, dissolved in 500 μL of water, the pH of the aqueous solution of L-arginine was adjusted to 11, and the aqueous solution of amino acid was...
Embodiment 2
[0050] Example 2 Preparation of triptolide succinate L-arginine amidomethyl ester
[0051] The preparation method of triptolide targeting prodrug comprises the following steps:
[0052] A 2 : Activation of triptolide succinate terminal carboxyl group
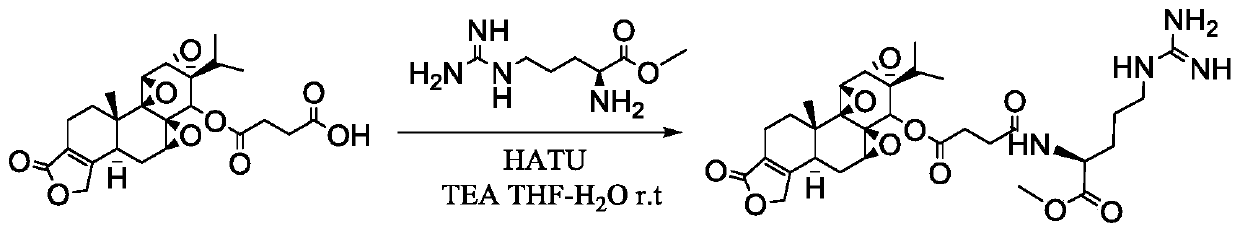
[0053] Such as figure 2 The preparation process shown. Use THF-water (v:v) = 10:1.5 as solvent, stirring and dissolving triptolide succinate with 1 part by weight, adding triethylamine with 2.02-4.06 parts by weight and stirring evenly, batchwise slowly Add 0.52-2.29 parts by weight of the activator, stir and activate at room temperature for 45min-1h, after TLC detects that the activation is complete, cool to -5°C to -30°C in a low-temperature reactor, and stir for later use.
[0054] B 2 : Active ester intermediate amidation stage
[0055] Another 25 mg (0.141 mmol) of L-arginine was weighed, dissolved in 500 μL of water, the pH of the aqueous solution of L-arginine was adjusted to 11, and the aqueous solution of amino a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com