Biological recombinant miR124-3p capable of effectively suppressing osteosarcoma growth
A recombinant, recombinant plasmid technology, applied in the field of genetic engineering, can solve problems such as side effects and limitations, and achieve the effects of high yield, low cost, and efficient aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] 【Example 1】Expressible htRNA Leu / miR124-3p and its positive control htRNA Leu Construction and amplification of recombinant plasmids
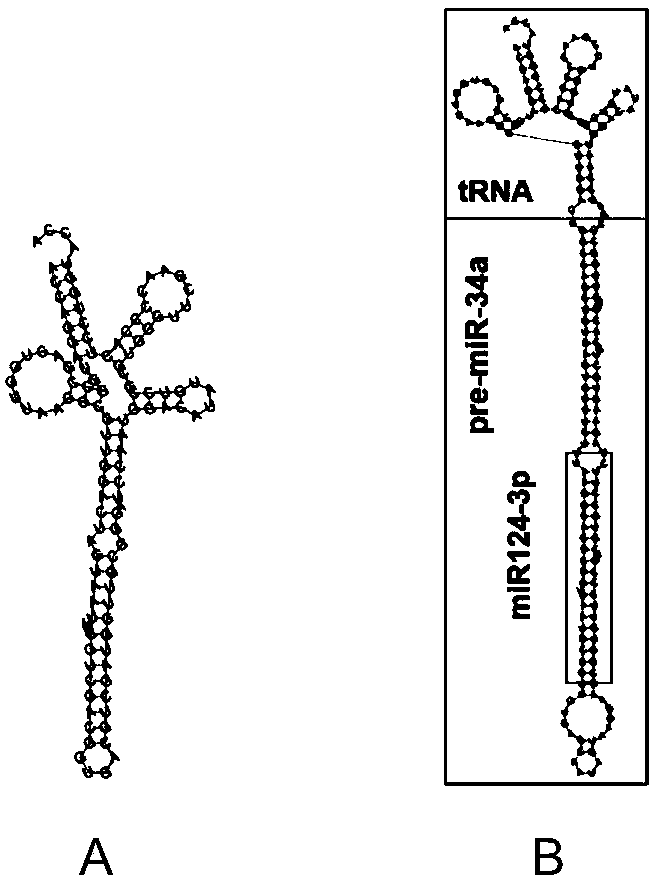
[0042] 1. htRNA Leu / miR124-3p and its positive control htRNA Leu Secondary Structure Design
[0043] Utilization of human-derived leucine (Leu)-carrying tRNA as htRNA Leu / miR124-3p main structure, design prediction of its secondary structure (such as figure 1 B) Through the professional RNA secondary structure design website: http: / / rna.tbi.univie.ac.at / cgi-bin / RNAWebSuite / RNAfold.cgi Sure. htRNA Leu / miR124-3p的碱基序列为:ACCAGGAUGGCCGAGUGGUUAAGGCGUUGGACUGGCCAGCUGUGAGUGUUUCUUUAAGGCACGCGGUGAAUGCCGUUGUGAGCAAUAGUAAGGAAGCGGUGUUCCCGUCGUGCCUUCUAGAAGUGCUGCACGUUGUUGGCCCGAUCCAAUGGACAUAUGUCCGCGUGGGUUCGAACCCCACUCCUGGUACCA。 positive control htRNA Leu The secondary structure of is designed in the same way (such as figure 1 A), its base sequence is: ACCAGGAUGGCCGAGUGGUUAAGGCGUUGGACUAGUAAUUUACGUCGACGGUGACGUCGAUGGUUGCGGGAUCCAAUGGACAUAUGUCC...
example 2
[0079] 【Example 2】htRNA Leu / miR124-3p and its positive control htRNA Leu purification of
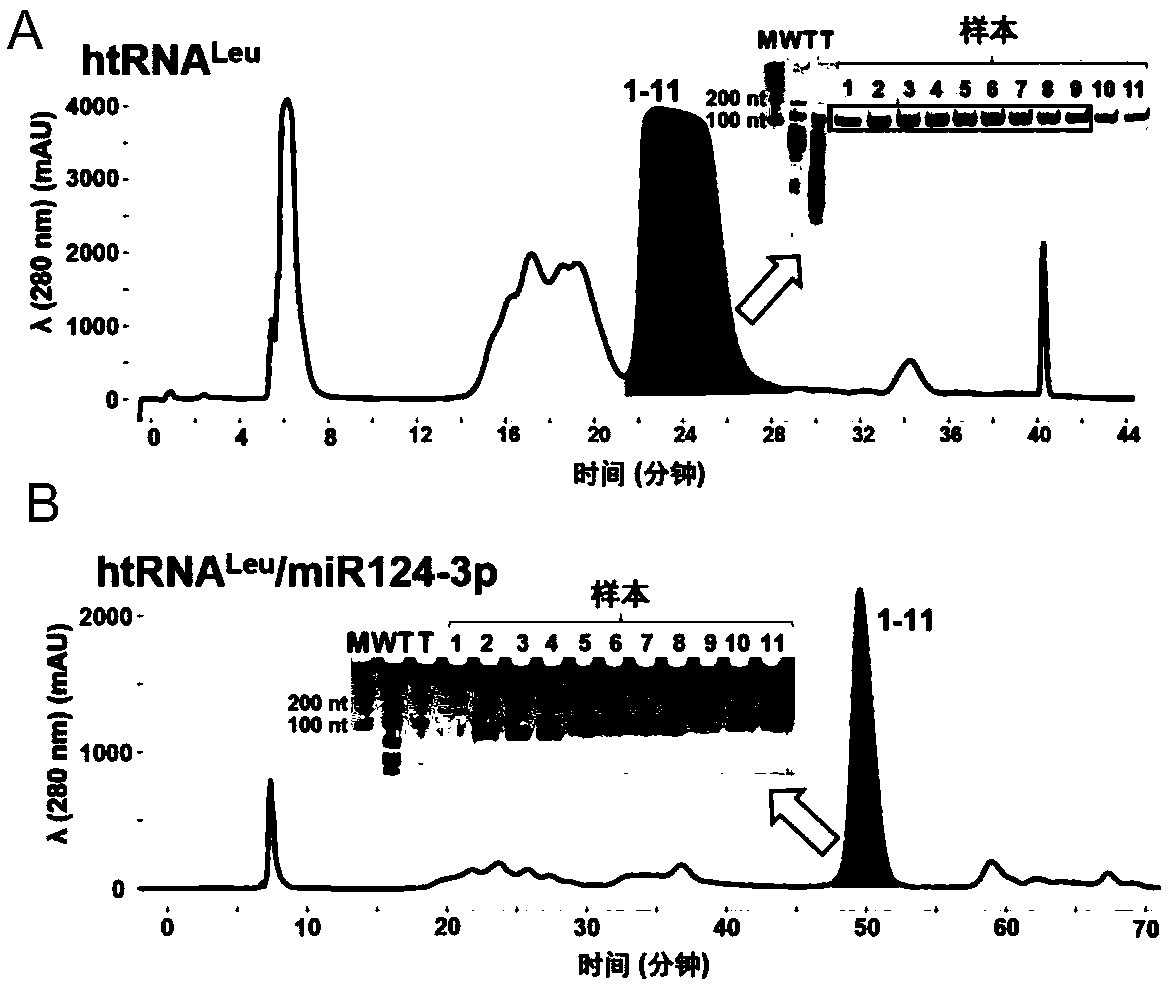
[0080] The filtered total RNA solution was purified using NGC Quest 10Plus fast protein liquid chromatography (FPLC) system (anion exchange column model and specification: ENrichTM Q 10×100), htRNA Leu / miR124-3p or its positive control htRNA Leu to separate (e.g. figure 2 B), the specific steps are as follows:
[0081] 1. Balance the ion exchange column, use 100% buffer A (10nM sodium phosphate, pH=7.0) to balance 2 volumes of ENrichTM Q 10×100 anion exchange column at a flow rate of 4.0ml / min;
[0082] 2. To elute the ion exchange column, use buffer B (10nM sodium phosphate + 1M sodium chloride, pH=7.0) with a gradient concentration of 0-55% to elute at a flow rate of 4.0ml / min for 30s, and then add buffer B The concentration of the solution was maintained at 55% for 2mins, and then the buffer B was increased from 55% to 65% for 10mins;
[0083] 3. Collection of htRNA Leu / miR...
example 3
[0096] 【Example 3】htRNA Leu / miR124-3p and its positive control htRNA Leu Purity analysis
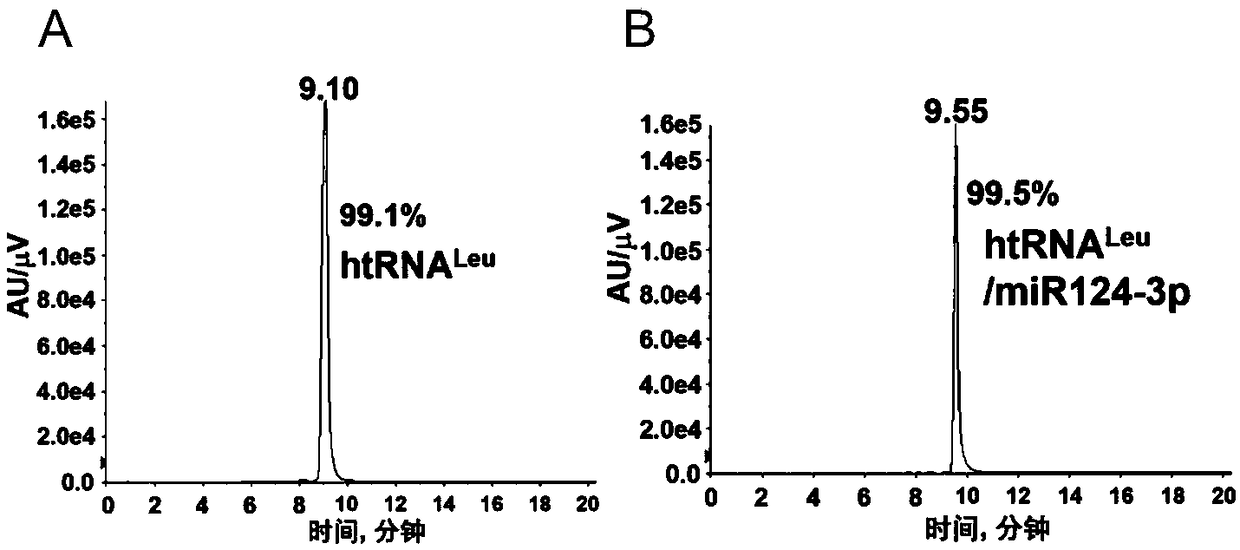
[0097] htRNA Leu / miR124-3p and its positive control htRNA Leu The purity of the Shimadzu LC-20AD high-performance liquid chromatography system is used for analysis (such as image 3 B), the chromatographic column model and specifications are: XBridge OST C18, 2.5μm, 10×50m, the buffer flow rate is 0.2ml / min, the column temperature is kept at 60°C, and the buffer A is: 8.6mM TEA and 100mM hexafluoroiso Aqueous propanol (pH=8.3), buffer B: 8.6 mM TEA and 100 mM hexafluoroisopropanol in methanol (pH=8.3).
[0098] 1.16% buffer B was passed through the column for 1min;
[0099] 2.22% buffer B was passed through the column for 1min;
[0100] 3. Photodiode array detector detects htRNA at 260nm Leu / miR124-3p or its positive control htRNA Leu waveform;
[0101] 4. Evaluation of final htRNA using peak area calculation Leu / miR124-3p and its positive control htRNA Leu Purity, the pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com