Glycosylnaphthalimide-containing fluorescent probe and application thereof
A technology based on naphthalimide and naphthalimide, applied in the field of novel glycosyl naphthalimide derivatives, which can solve the problems of low solubility, measurement results easily affected by the environment, and the inability to visually evaluate enzyme activity, etc. problem, to achieve the effect of high research value, excellent optical and physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
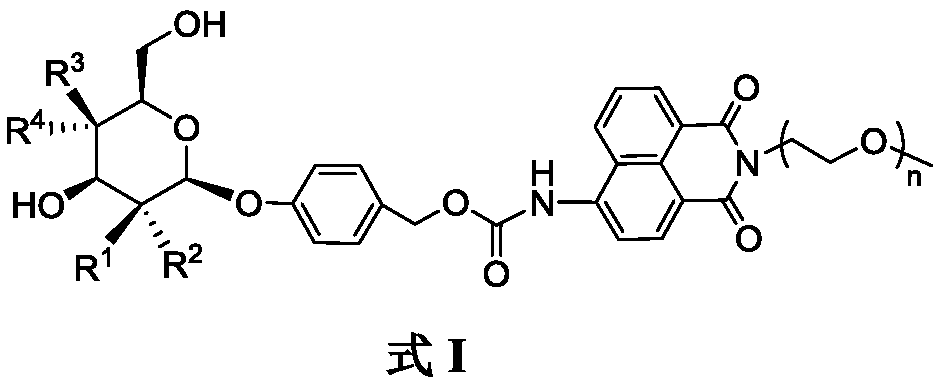
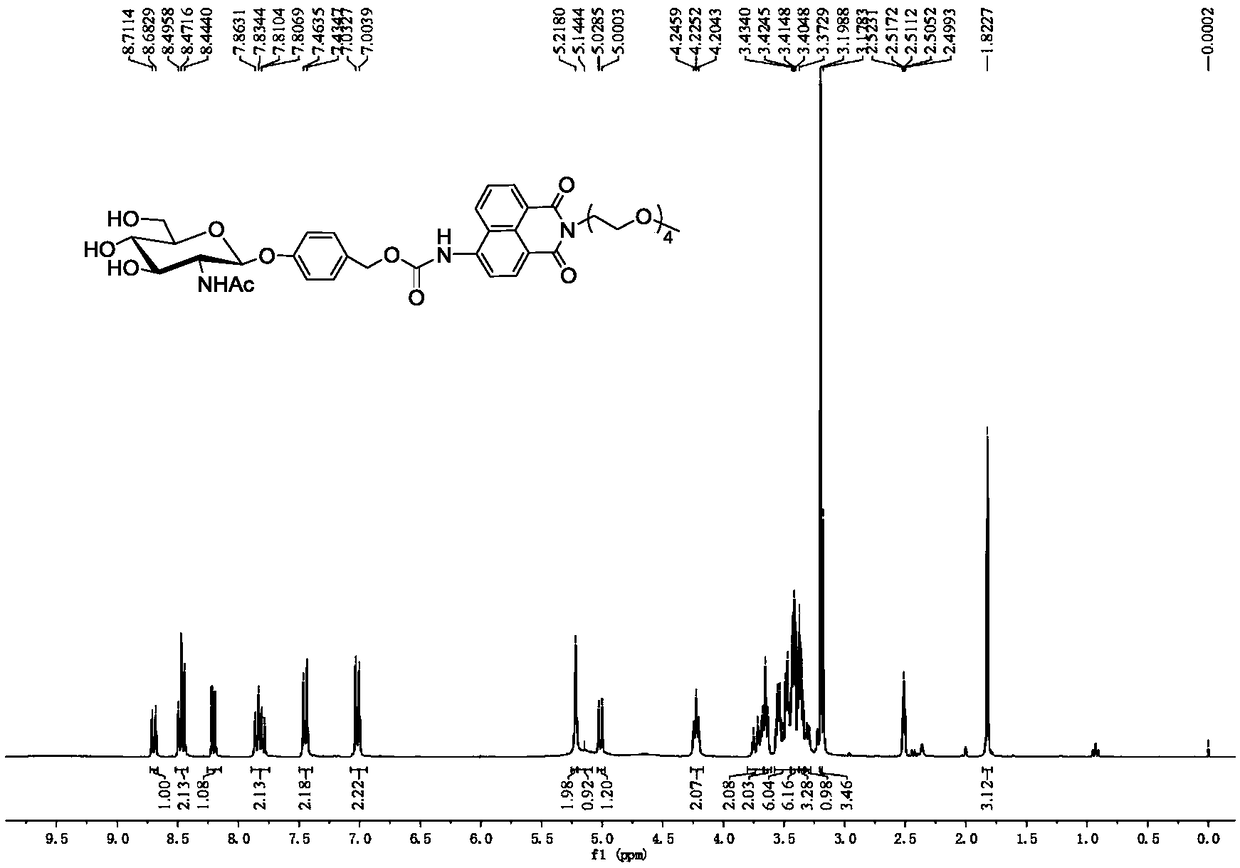
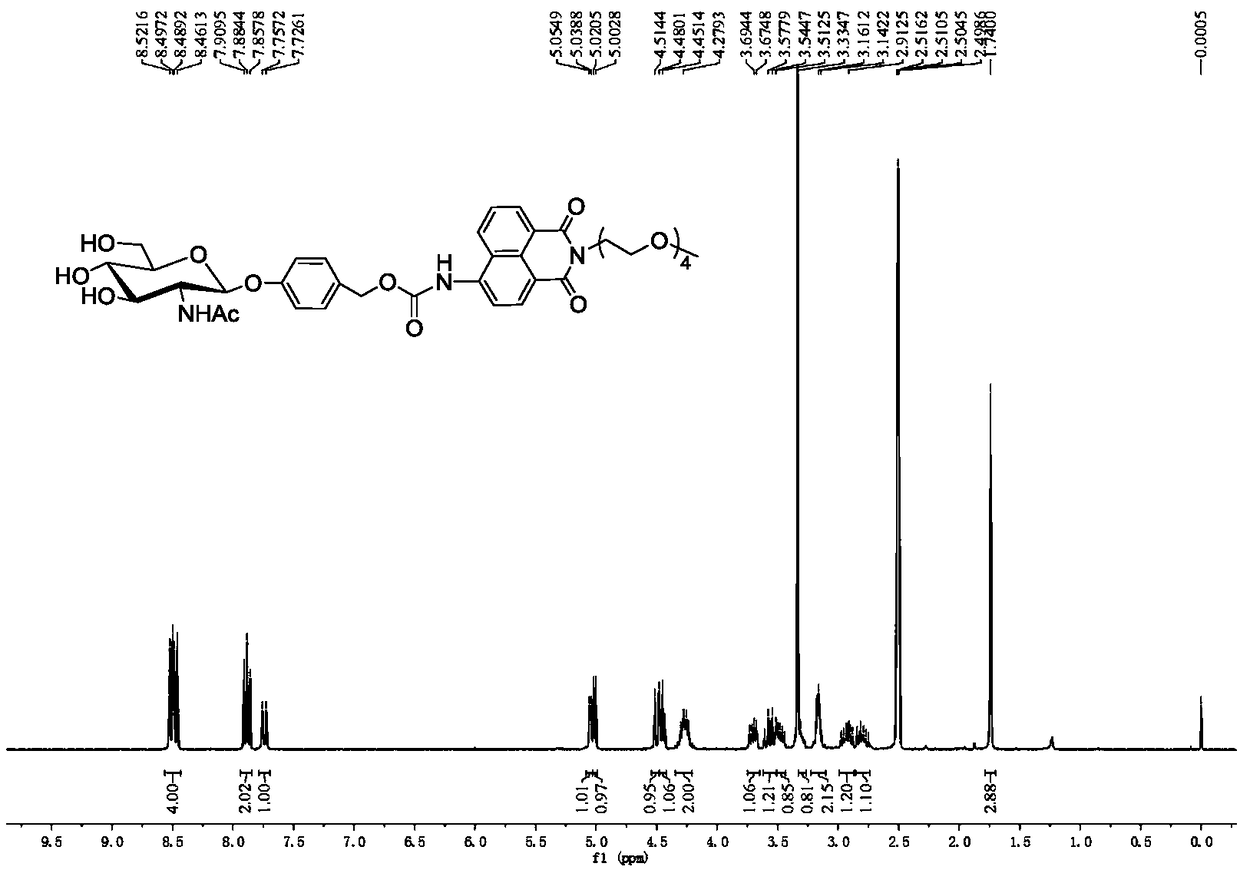
[0024] Embodiment 1, R in formula I 1 =H,R 2 =NHAc,R 3 =H,R 4 =OH, the compound preparation of n=4, concrete steps are as follows:
[0025]
[0026] (1) Add N-acetylglucosamine (10g, 40.3mmol) and acetyl chloride (40ml, 186.8mmol) into a 250mL round-bottomed flask, react at room temperature for 48h, and monitor the completion of the reaction by TLC. The reaction solution was added to 500mL ice water, extracted with 200mL DCM to obtain an organic layer, and then saturated NaHCO 3 Aqueous solution (100mL), water (100mL) washing, anhydrous Na 2 SO 4 After drying and concentration, 2-acetylamino-3,4,6-tri-O-acetyl-2-deoxy-α-D-glucopyranoside chloride was obtained, which was directly carried out to the next reaction without further purification.
[0027] Add 4-hydroxybenzaldehyde (2.5g, 20.5mmol) in the reaction bottle of 500mL, Na 2 CO 3 (2.2g, 20.5mmol), tetrabutylammonium bromide (6.6g, 20.5mmol), 100mL DCM and 150mL H 2 O, 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-...
Embodiment 3
[0038] The effect of embodiment 3 compound I and Hex and the relation of time
[0039]On the basis of Example 2, we studied the relationship between the action of Compound I and Hex and time. Add 160 μL of 200 μM compound I and 1440 μL of PBS buffer solution containing Hex to the cuvette in sequence, and measure the change of the fluorescence spectrum of the reaction in 0-80 minutes at room temperature. We found that the fluorescence intensity with a wavelength of 438nm has been decreasing in the first 10 minutes, and a new fluorescence emission peak was added at 540nm, and the fluorescence emission peak at 540nm increased rapidly in the last 70 minutes, far greater than the fluorescence intensity at 438nm. It shows that the probe and the enzyme have a good response over time.
Embodiment 4
[0040] The kinetic parameter of embodiment 4 compound I and Hex
[0041] Further, we explored the kinetic parameters of compound I and Hex. First configure a series of concentrations of Compound I solutions in a cuvette, measure the relationship between its fluorescence intensity and concentration, and obtain y=166.95x-1.5388 (R 2 =0.9994), and then configure compound I solutions with different concentrations containing Hex, incubate at 37° C. for 10 minutes, and measure the fluorescence value. Get the linear curve y=16.18x+0.40677(R 2 =0.9995), and then get K m =39.76nm( Figure 4 ), indicating that compound I has a good affinity with the enzyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com