Aliphatic-series hyperbranched epoxy resin and preparation method thereof
An epoxy resin and aliphatic technology, applied in the field of aliphatic hyperbranched epoxy resin and its preparation, can solve the problems of long synthetic route of hyperbranched epoxy resin, limited types of monomers, difficult structure design, etc. The effect of flexibility and chemical stability, less impurities and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
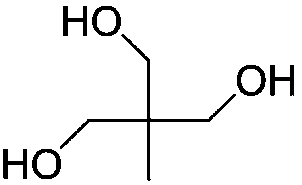
[0035] The structural formula of the polyol compound used is
[0036]
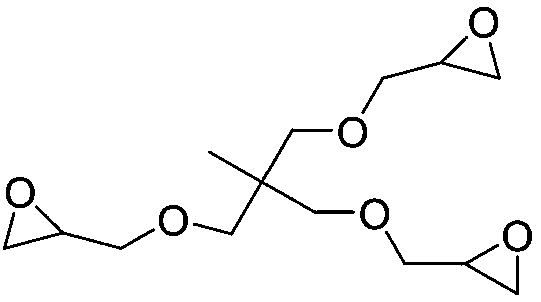
[0037] The structural formula of the epoxy compound used is
[0038]
[0039] S1: Add 12g (0.1mol) trihydroxymethylethane and 90.6g (0.3mol) trimethylolpropane triglycidyl ether into the reactor, and add 4.17g (0.015mol) tetrabutyl chloride to it ammonium chloride. Under the protection of a helium atmosphere, the temperature was slowly raised to 120° C., and cooled after 12 hours of reaction.
[0040] S2: Add 200 mL of tetrahydrofuran to dissolve the product. After the product is completely dissolved, pour it into deionized water while stirring, and settle for 3 hours to separate layers.
[0041] S3: Take the lower layer liquid and add 10 g of anhydrous sodium sulfate, dry for 12 hours, filter, and distill the solution under reduced pressure to obtain the aliphatic hyperbranched epoxy resin.
Embodiment 2
[0043] The structural formula of the polyol compound used is
[0044]
[0045] The structural formula of the epoxy compound used is
[0046]
[0047] S1: 12 g (0.1 mol) of trihydroxymethylethane and 108 g (0.3 mol) of pentaerythritol glycidyl ether were added to a reactor, and 8.34 g (0.03 mol) of tetrabutylammonium chloride was added thereto. Under the protection of a helium atmosphere, the temperature was slowly raised to 120° C., and cooled after 12 hours of reaction.
[0048]S2: Add 200 mL of tetrahydrofuran to dissolve the product. After the product is completely dissolved, pour it into deionized water while stirring, and settle for 3 hours to separate layers.
[0049] S3: Take the lower layer liquid and add 10 g of anhydrous sodium sulfate, dry for 12 hours, filter, and distill the solution under reduced pressure to obtain the aliphatic hyperbranched epoxy resin.
Embodiment 3
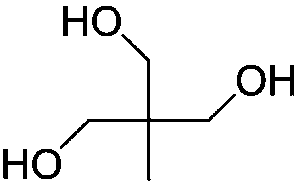
[0051] The structural formula of the polyol compound used is
[0052]
[0053] The structural formula of the epoxy compound used is
[0054]
[0055] S1: 13.4 g (0.1 mol) of trihydroxymethylpropane and 36 g (0.1 mol) of pentaerythritol glycidyl ether were added to a reactor, and 0.278 g (0.001 mol) of tetrabutylammonium chloride was added thereto. Under the protection of a helium atmosphere, the temperature was slowly raised to 150° C., and cooled after 8 hours of reaction.
[0056] S2: Add 100 mL of tetrahydrofuran to dissolve the product. After the product is completely dissolved, pour it into deionized water while stirring, and settle for 3 hours to separate layers.
[0057] S3: Take the lower layer liquid and add 5 g of anhydrous sodium sulfate, dry for 12 hours, filter, and distill the solution under reduced pressure to obtain the aliphatic hyperbranched epoxy resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com