Perfluoropolyether group-containing silane compound, preparation method thereof, surface treatment agent and article
A silane compound, perfluoropolyether-based technology, applied in polyether coatings, biocide-containing paints, coatings, etc., can solve the problems of high production cost, high price, difficult synthesis process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0199] The present invention also provides the preparation method of the silane compound containing perfluoropolyether group shown in aforementioned formula (2), described method comprises:
[0200] The formula is Rf-CH 2 The compound of -O-X-COOH is converted into the silane compound containing perfluoropolyether group of formula (2) with acid halide reagent and aminosilane coupling agent,
[0201]
[0202] Among them, R f for:
[0203] q, r and s are independently integers ranging from 0 to 200, the sum of q, r and s is at least 1, and the order of existence of the repeating units enclosed in brackets marking q, r or s is arbitrary in the formula, m is an integer of 1 to 6, t is 0 or 1, Z is a fluorine atom or a trifluoromethyl group,
[0204] X is a divalent organic group,
[0205] T independently in each occurrence is hydroxyl, hydrolyzable group or hydrocarbyl,
[0206] Each time Q appears independently, it is -Y-SiR 1 j R 2 3-j ,
[0207] Each time Y appea...
Embodiment approach
[0274] According to a more preferred embodiment of the present invention, the preparation method for preparing the silane compound containing perfluoropolyether group comprises the following steps:
[0275] Step 1: Let the formula be Rf-CH 2 The compound of OH first reacts with potassium hydroxide at room temperature, and then reacts with the formula BrCH 2 COOC 4 h 9 The compound undergoes a nucleophilic substitution reaction at room temperature or under heating (preferably 25-75° C.) to obtain the formula Rf-CH 2 -OCH 2 COOC 4 h 9 Ester-based perfluoropolyether compounds,
[0276]
[0277] Rf is CF 3 (OCF 2 CF 2 ) r (OCF 2 ) s OCF 2 -, r+s is 35-85, and its number average molecular weight is 3000-8000;
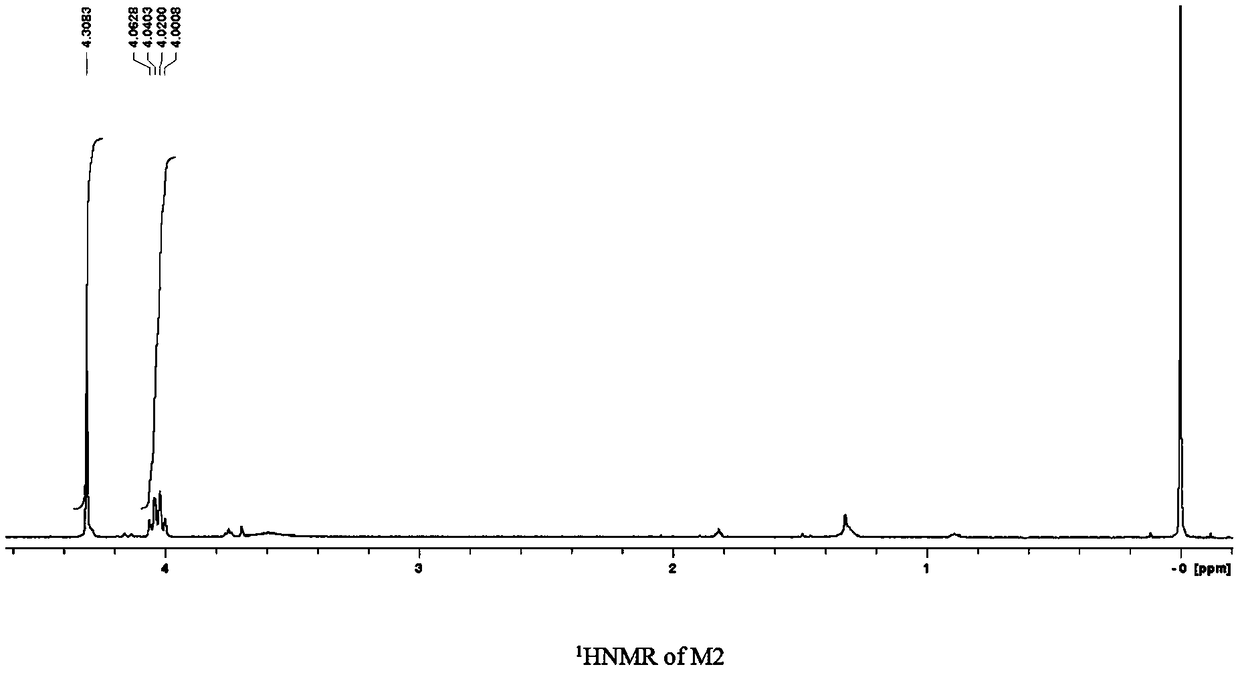
[0278] Step 2: The formula is Rf-CH 2 -OCH 2 COOC 4 h 9 The ester-based perfluoropolyether compound reacts with alkali to hydrolyze, and after adding hydrochloric acid to adjust the acidic separation, the formula is Rf-CH 2 -O-CH 2 COOH carboxyl perflu...
Synthetic example 1
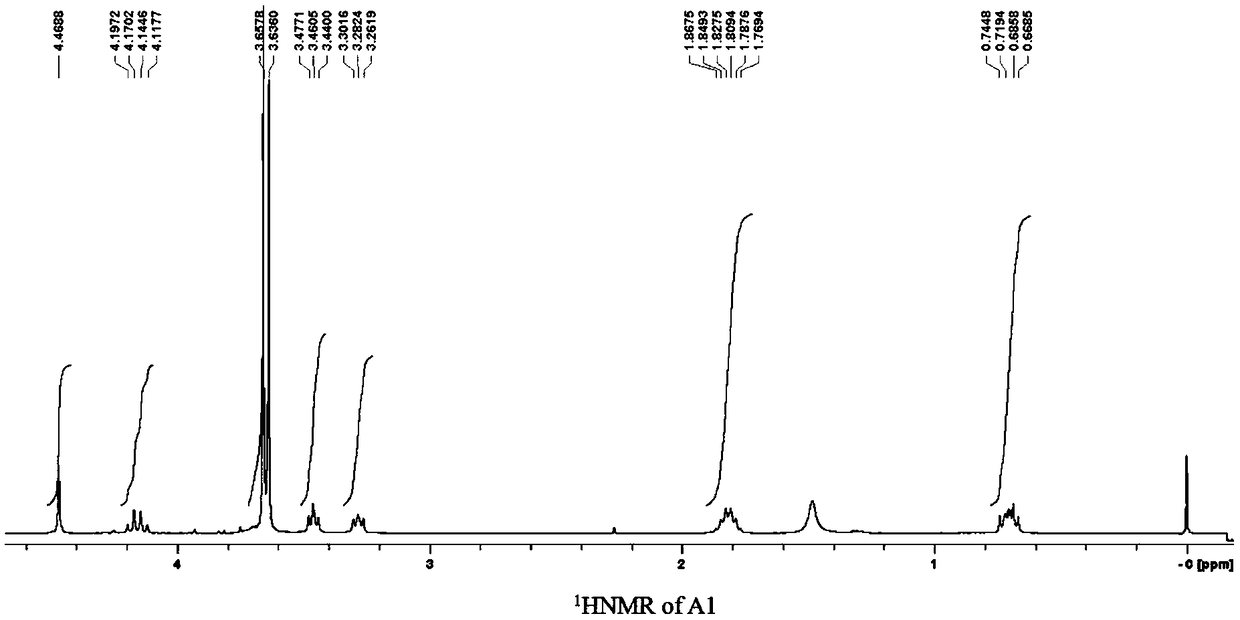
[0294] The silane compound A1 containing perfluoropolyether group was synthesized according to the following steps
[0295] step 1:
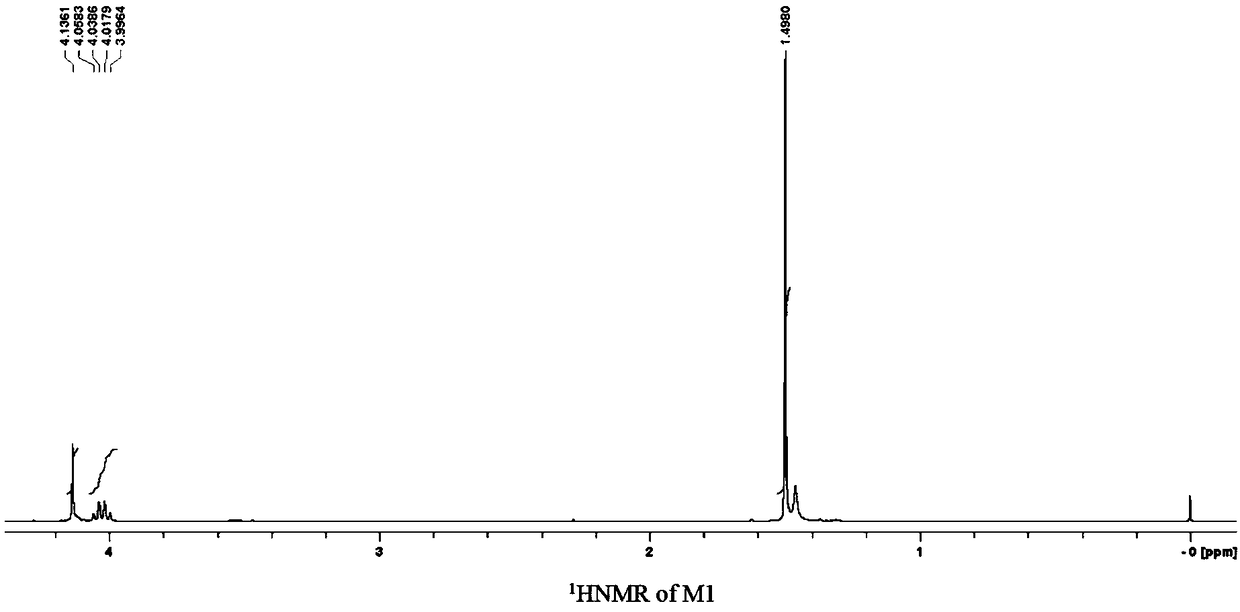
[0296] In a 100-mL three-necked round-bottom flask equipped with a stirrer, add 10 g of the average composition as CF 3 (OCF 2 CF 2 ) r (OCF 2 ) s OCF 2 CH 2 OH (the sum of r and s is 35-42) perfluoropolyether modified alcohol (number average molecular weight is 3500-4000), 15mL 1,3-bis(trifluoromethyl)benzene and 5mL ethylene glycol dimethyl Ether and 2.6 g of 50 wt % potassium hydroxide solution were stirred at room temperature for 3 hours. Then, 3.8 mL of tert-butyl bromoacetate and 0.42 g of tetrabutylammonium bromide were sequentially added to the reaction flask, and stirred at 50° C. for 5 hours. After water extraction and vacuum distillation, 9.6 g of a colorless and transparent product was obtained, which was the ester-based perfluoropolyether compound (M1): CF 3 (OCF 2 CF 2 ) r (OCF 2 ) s OCF 2 CH 2 OCH 2 COOC 4 h 9 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water contact angle | aaaaa | aaaaa |
| Load | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com