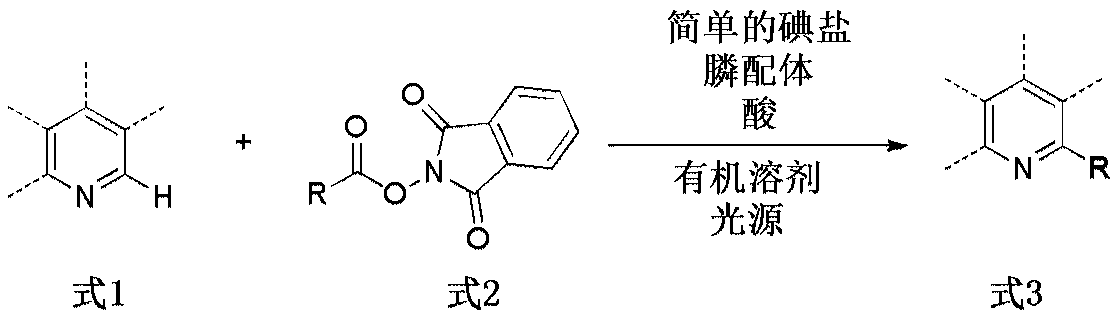
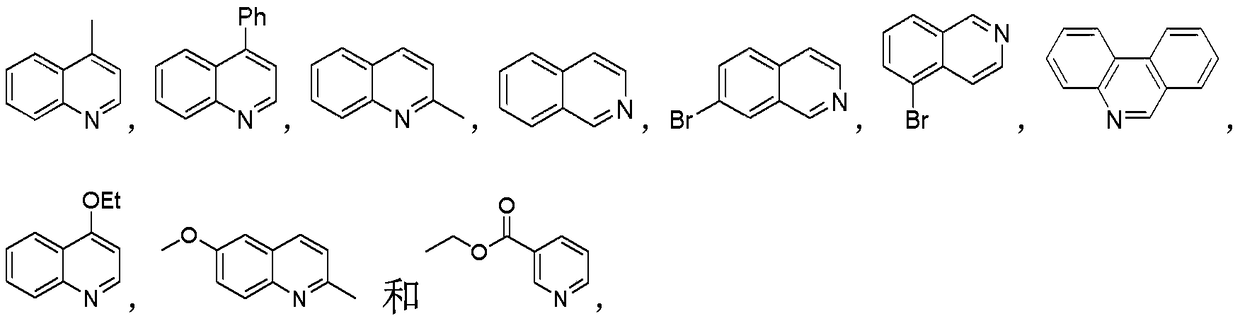
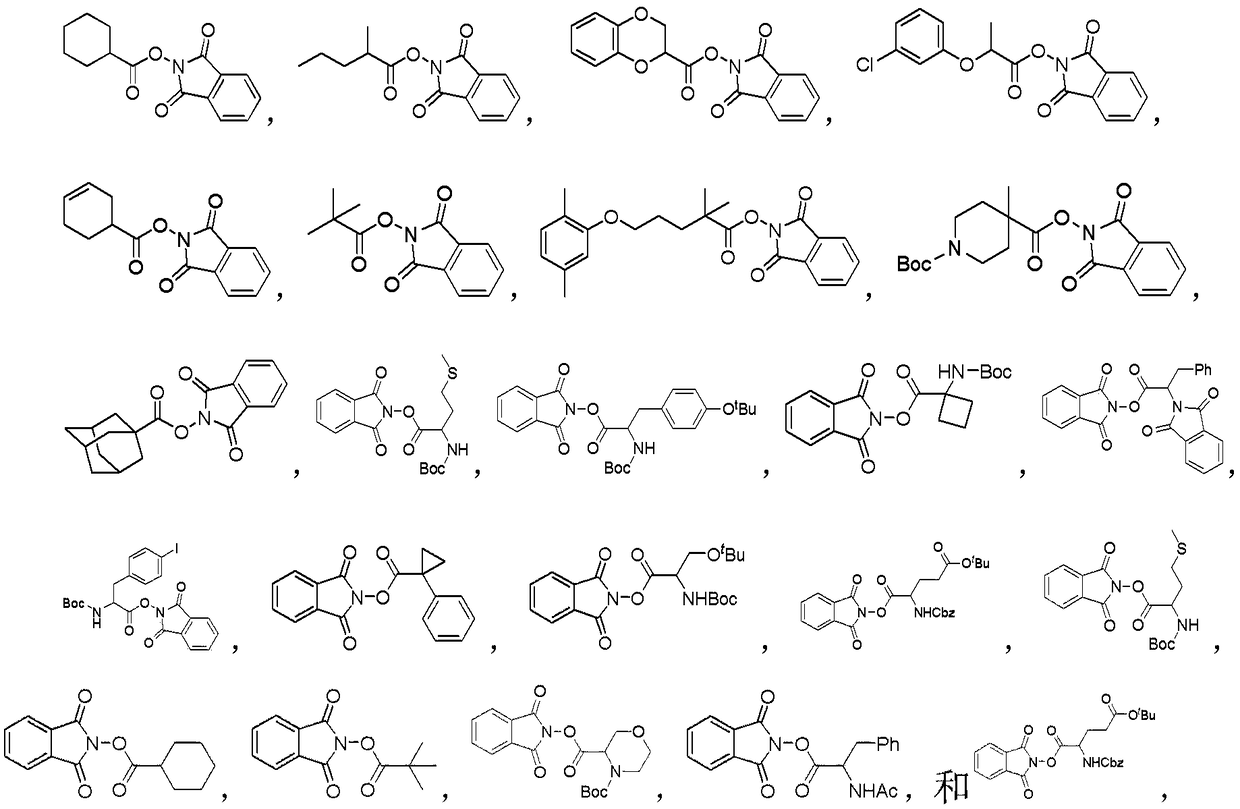
Method for catalyzing decarboxylation of active carboxylic ester to introduce nitrogen heterocycle through photo-induced non-metal
A carboxylate, light-induced technology, applied in organic chemistry and other directions, can solve the problems of high price of iridium, increased production cost, easy residue, etc., and achieve the effect of large synthetic value prospect, substrate scope and good functional group compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1, preparation 2-cyclohexyl-4-methylquinoline
[0056] Reaction formula:
[0057] (where Cy represents cyclohexyl)
[0058] The specific method is as follows:
[0059] Add NaI (0.02mmol (that is, 10mol% of the nitrogen-containing heterocyclic compound 1a. ), 3mg), PPh 3 (0.04mmol (that is, 20mol% of the nitrogen-containing heterocyclic compound 1a. The same below), 10.5mg) and 1,3-dioxoisoindolin-2-ylcyclohexanecarboxylate (2a, 0.3 mmol, 81.9 mg). The air in the tube was completely replaced with argon three times, and then 2 mL of acetone, trifluoroacetic acid (0.2 mmol, 22.8 mg), and 4-methylquinoline (1a, 0.2 mmol, 28.6 mg) were added under an argon atmosphere. The reaction system was continuously stirred for 15 hours at room temperature under the irradiation of a blue LED lamp (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction is complete, use H 2 The reaction was quenched with O, and the reaction solution was extra...
Embodiment 2
[0062] Embodiment 2, preparation 4-methyl-2-(pent-2-yl) quinoline
[0063] Reaction formula:
[0064]
[0065] The specific method is as follows:
[0066] Add NaI (10mol%, 3 mg) and PPh into a 10mL Schlenk reaction tube (Beijing Shinwell Glass Instrument Co., Ltd., F891410 reaction tube, capacity 10mL, ground 14 / 20) 3 (20mol%, 10.5mg) and 1,3-dioxoisoindolin-2-yl 2-methylpentanoate (0.3mmol, 78.3mg). The air in the tube was completely replaced with argon three times, and then 2 mL of acetonitrile, trifluoroacetic acid (0.2 mmol, 22.8 mg), and 4-methylquinoline (0.2 mmol, 28.6 mg) were added under an argon atmosphere. The reaction system was continuously stirred for 17 hours at room temperature under the irradiation of a blue LED lamp (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction is complete, use H 2 The reaction was quenched with O, and the reaction solution was extracted with ethyl acetate (3*10 mL), and the combined organic phase w...
Embodiment 3
[0070] Example 3, preparation of 2-(2,3-dihydrobenzo[b][1,4]dioxin-2-yl)-4-methylquinoline
[0071] Reaction formula:
[0072]
[0073] The specific method is as follows:
[0074] Add NaI (20mol%, 6 mg), PPh into a 10mL Schlenk reaction tube (Beijing Xinweier Glass Instrument Co., Ltd., F891410 reaction tube, capacity 10mL, ground 14 / 20) 3(20mol%, 10.5mg) and 1,3-dioxoisoindolin-2-yl 2,3-dihydrobenzo[b][1,4]dioxin-2-carboxylate (0.3 mmol, 97.5mg). The air in the tube was completely replaced with argon three times, and then 2 mL of N,N-dimethylacetamide, trifluoroacetic acid (0.2 mmol, 22.8 mg), 4-methylquinoline (0.2 mmol, 28.6 mg) were added under an argon atmosphere. ). The reaction system was continuously stirred for 17 hours at room temperature under the irradiation of a blue LED lamp (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction is complete, use H 2 The reaction was quenched with O, and the reaction solution was extracted with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com