Fluorescent material and preparation method and application thereof
A fluorescent material and fluorescent technology, applied in the field of organic fluorescent materials, can solve problems such as difficult to use, single color change, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
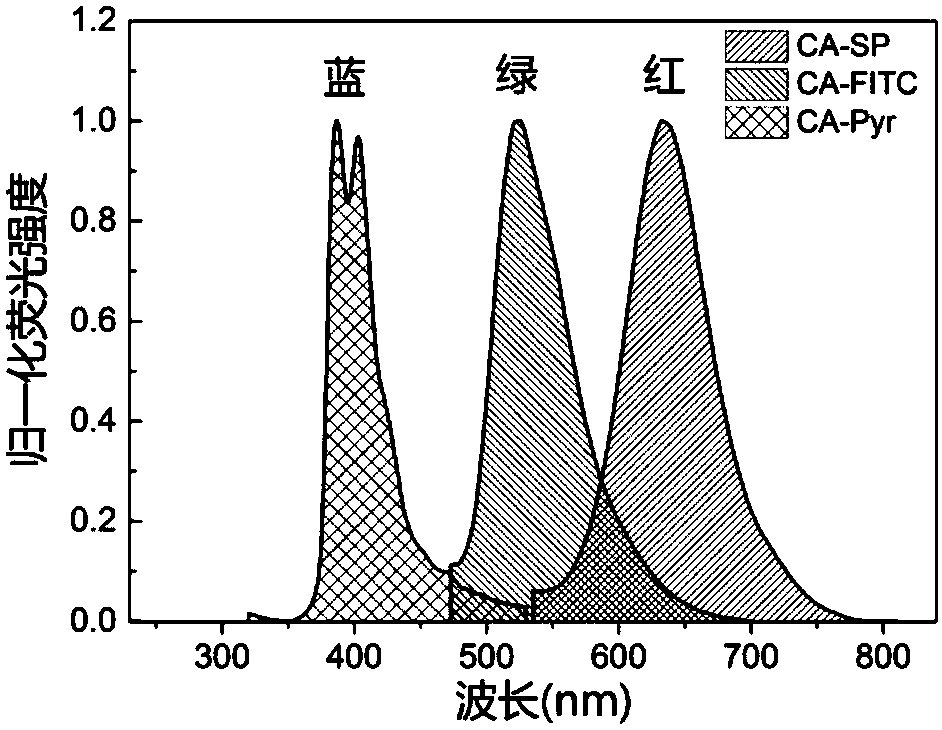
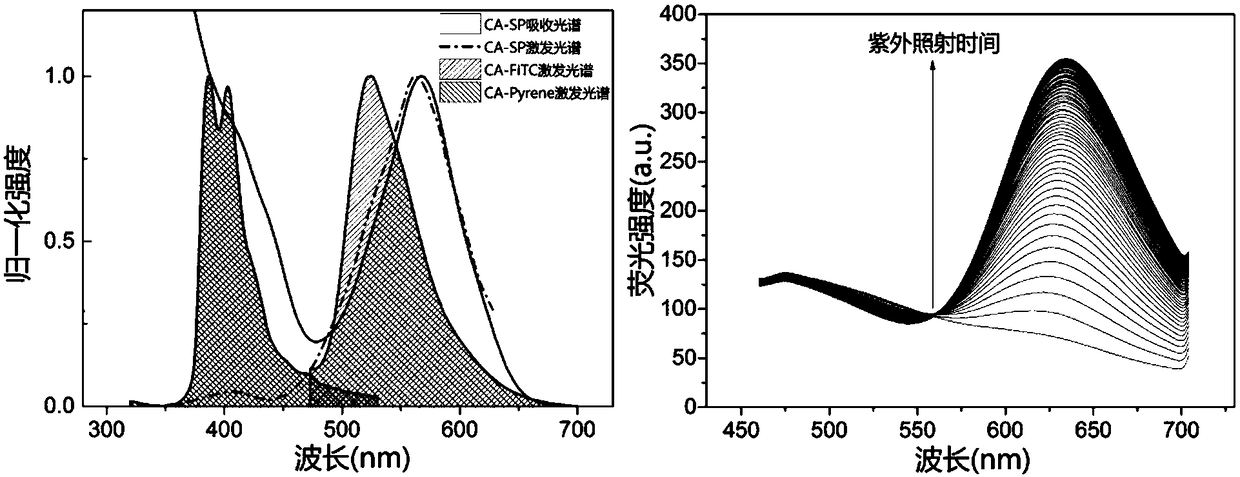
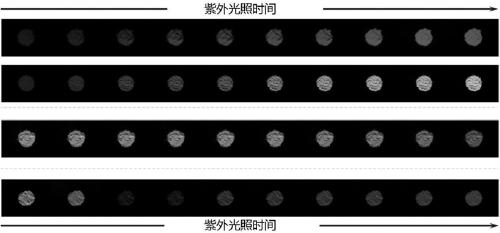
[0069] Preparation of Red Fluorescent Modified Polysaccharide and Its Derivative CA-SP
[0070] 2.70g cellulose acetate (CA 2.55 ) and 0.19g SPCOOH were dissolved with 80mL N,N-dimethylformamide (DMF), then 2.06g dicyclohexylcarbodiimide (DCC) and 1.21g 4-dimethylaminopyridine (DMAP ) as a catalyst. The mixed solution was refluxed at 155°C for 3 hours and then stopped, then the mixed solution was poured into deionized water for precipitation, dried and dissolved with DMF, and then deionized water was used for precipitation, and this was repeated 2-3 times, and finally the filter cake After lyophilization, 2.9 g of CA-SP was obtained as a dark red powder with a yield of 88%.
[0071] The CA-SP is a modified ester of cellulose acetate followed by spiropyran, and the degree of substitution of spiropyran is 0.07.
preparation example 2
[0073] Preparation of Red Fluorescent Modified Polysaccharide and Its Derivative CA-SP'
[0074] 2.70g cellulose acetate (CA 2.55 ) and 0.048g SPCOOH were dissolved with 80mL N,N-dimethylformamide (DMF), then 2.06g dicyclohexylcarbodiimide (DCC) and 1.21g 4-dimethylaminopyridine (DMAP ) as a catalyst. The mixed solution was refluxed at 155°C for 3 hours and then stopped, then the mixed solution was poured into deionized water for precipitation, dried and dissolved with DMF, and then deionized water was used for precipitation, and this was repeated 2-3 times, and finally the filter cake After lyophilization, 2.9 g of dark red powder CA-SP' was obtained, with a yield of 88%.
[0075] The CA-SP' is a modified ester of cellulose acetate followed by spiropyran, and the degree of substitution of spiropyran is 0.02.
preparation example 3
[0077] Preparation of Green Fluorescent Modified Polysaccharide and Its Derivative CA-FITC
[0078] 3.0g cellulose acetate (CA 2.55 ) into a 250mL round-bottomed Schlenk reaction flask, operated anhydrous and anaerobic (inflated with nitrogen after vacuuming) three times, and pipetted 60mL of anhydrous DMF ( Molecular Preservation) dissolves. Add 0.1298g of fluorescein isothiocyanate (FITC) into a 100mL Schlenk tube for three anaerobic operation with water, then pipette 20mL of anhydrous DMF for dissolution. The dissolved FITC solution was transferred to the CA solution, 50 μL of dibutyltin dilaurate (DBTDL) was added, and the mixed solution was reacted at 100° C. for 4 h to stop the reaction. The reactant was directly poured into methanol for precipitation, the filter cake was rinsed with a small amount of ether, dried and then dissolved in DMF, poured into deionized water for precipitation, filtered, and freeze-dried to obtain 2.80 g of CA-FITC as a light yellow powder. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com