Hydrogel compound, preparation method and application
A composite and hydrogel technology, which is applied in the field of biomedical engineering, can solve the problems of poor matrix integration, poorer performance than hyaline cartilage, and short cell life, and achieve the effects of rapid gelation, increased in vivo stability, and less trauma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
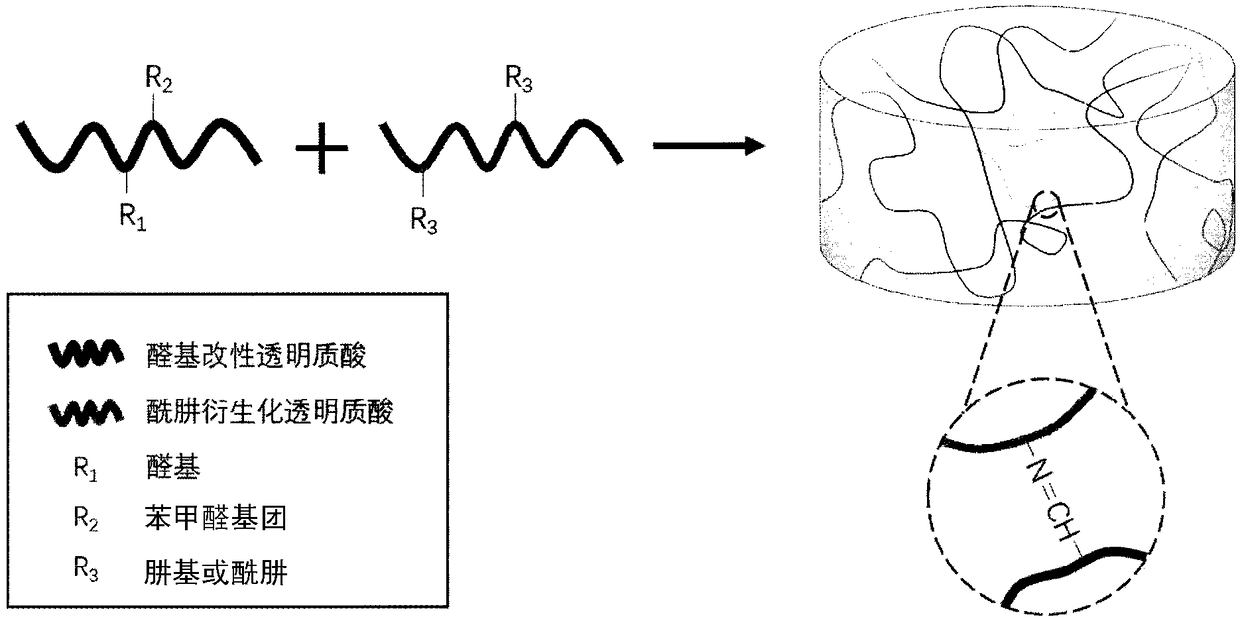
[0067] Preparation of aldehyde-modified hyaluronic acid
[0068] The aldehyde-modified hyaluronic acid of the present invention can be prepared by the following two methods:
[0069] The first method: oxidizing hyaluronic acid to open the ring to obtain aldehyde groups, and then obtain aldehyde group-modified hyaluronic acid (I). According to the molar ratio of HA to oxidizing agent (1:1)~(1:10), add oxidizing agent into hyaluronic acid solution (HA), and put the system in dark place to react for 0.5~6 hours. Ethylene glycol was added to remove excess oxidant, and after stirring was continued for 1 hour, the product was collected, purified and dried to obtain aldehyde-modified hyaluronic acid (I). The oxidizing agent may be one of periodate, permanganate, chlorate and the like.
[0070] The second method:
[0071] According to the above-mentioned first method to aldehyde-modified hyaluronic acid (I), on the basis of aldehyde-modified hyaluronic acid (I), carry out modif...
Embodiment 1
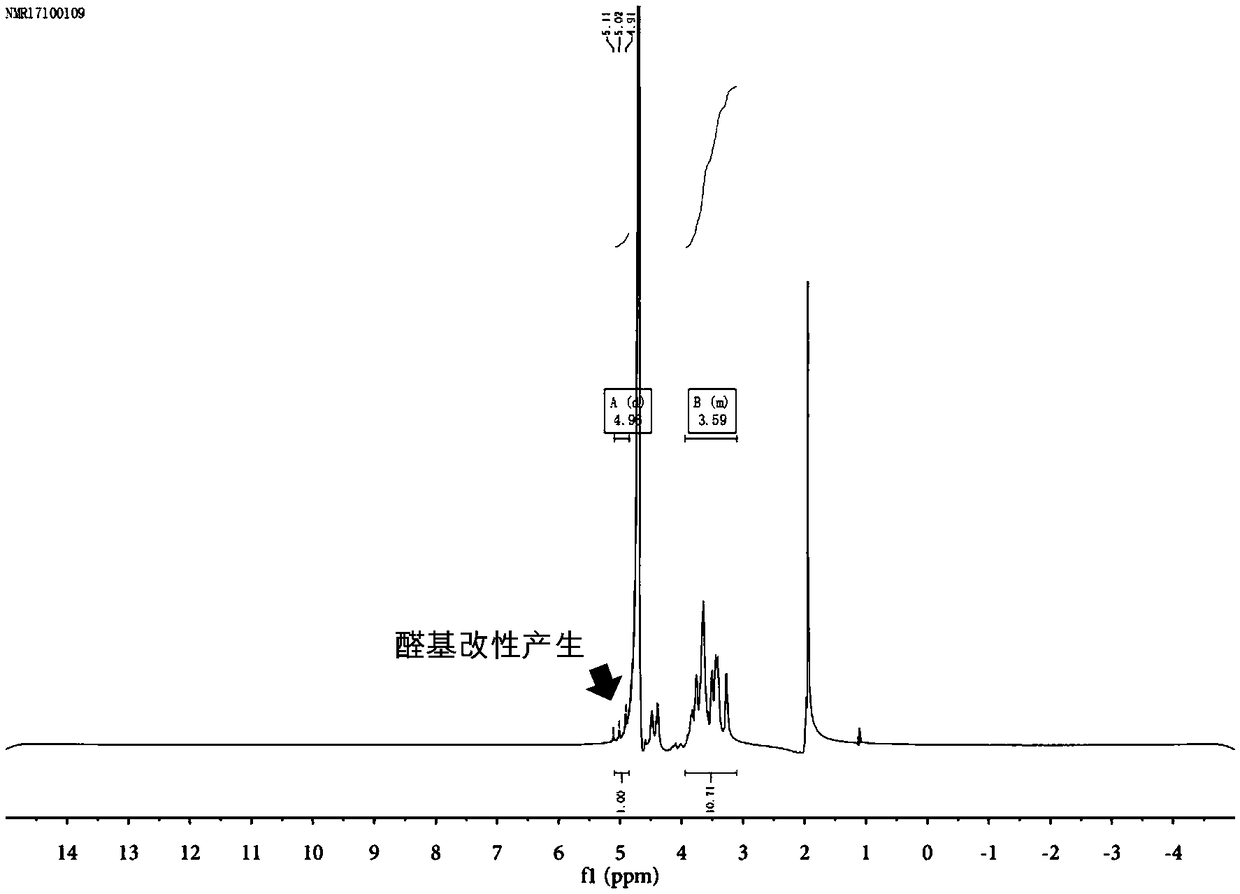
[0083] Sodium hyaluronate with a molecular weight of 100,000 to 200,000 Daltons was dissolved in water at room temperature to form a 0.01 g / mL hyaluronic acid solution. Add cation exchange resin and stir overnight, and filter to obtain hyaluronic acid solution (HA). Add 5mL of 0.5M sodium periodate solution to 100mL of 0.01g / mL hyaluronic acid solution for oxidation, and place the system in the dark for 3 hours to react. Add 0.5mL ethylene glycol to remove excess sodium periodate, continue stirring for 1 hour, collect the product, dialyze with a dialysis bag with a molecular weight cut-off of 3500 Daltons for three days, and freeze-dry to obtain aldehyde-modified hyaluronic acid (HA- A). The degree of substitution of the aldehyde group is 37% (such as figure 2 shown).
Embodiment 2
[0085] Sodium hyaluronate with a molecular weight of 100,000-200,000 Daltons is used to dissolve in water at room temperature to form a 0.03 g / mL hyaluronic acid solution. Add cation exchange resin and stir overnight, and filter to obtain hyaluronic acid solution (HA). Add 15 mL of 5M sodium periodate solution to 100 mL of 0.03 g / mL hyaluronic acid solution for oxidation, and place the system in the dark for 0.5 hours to react. Add 10 mL of ethylene glycol to remove excess sodium periodate, continue stirring for 1 hour, collect the product, dialyze for three days with a dialysis bag with a molecular weight cut-off of 3500 Daltons, and freeze-dry to obtain aldehyde-modified hyaluronic acid (HA-A ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com