Preparation of GrapheneConducting Polymer Composite Electrode Materials by Mechanochemical Polymerization
A conductive polymer and mechanochemical technology, applied in the field of graphene/conductive polymer composite electrode materials prepared by mechanochemical polymerization, can solve the problem of poor dispersion of graphene and polyaniline, affecting the electrochemical performance of composite materials, and easy regeneration of graphene. Agglomeration and other problems, to achieve the effects of excellent electrochemical performance, controllable loading content, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first embodiment, the graphene / polyaniline composite electrode material was prepared by mechanochemical polymerization.
[0023] Preparation:
[0024] (1) Add 0.2 g of natural graphite with a size of 50 mesh and 9.8 g of aniline into 100 mL of 10 wt.% dilute hydrochloric acid aqueous solution as liquid A.
[0025] (2) Dissolve 25 g of ammonium persulfate, 0.1 g of sodium lignosulfonate, and 1 mL of ethanol in 100 mL of deionized water, as liquid B.
[0026] (3) Liquid A and liquid B were mixed uniformly and then transferred to a ball milling reactor, and reacted by mechanical ball milling at 300 rpm for 4 hours to obtain mixture C.
[0027] (4) Mixture C was washed and filtered with 100 mL deionized water to obtain solid D.
[0028] (5) The solid D was washed and filtered with 100 mL of ethanol, and vacuum-dried at 60° C. to obtain a graphene / polyaniline composite electrode material.
[0029] Performance Characterization:
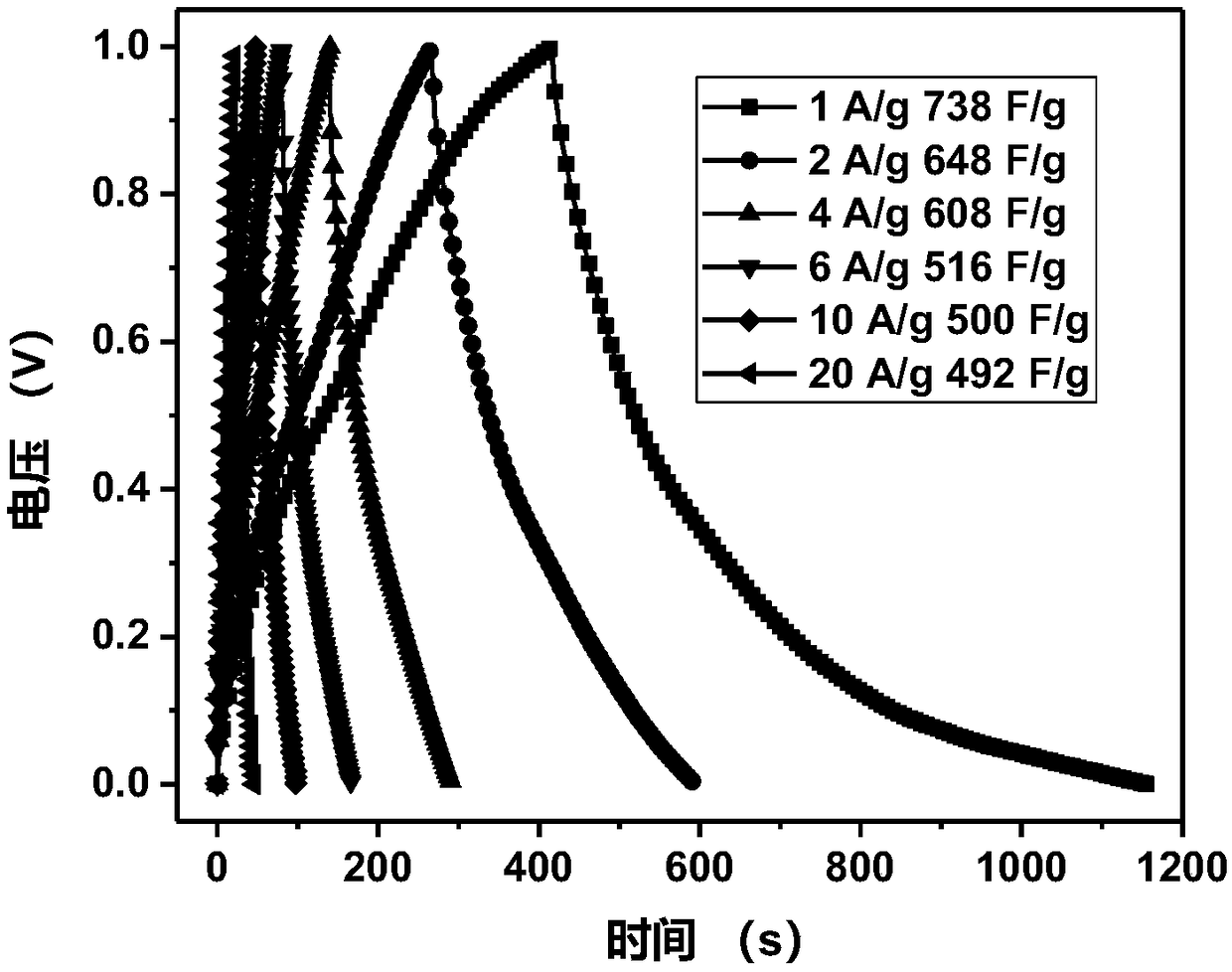
[0030] The resulting graphene / polya...
Embodiment 2
[0033] In the second embodiment, the graphene / polypyrrole composite electrode material is prepared by mechanochemical polymerization, which specifically includes the following steps:
[0034] (1) Take 0.5 g of flake graphite with a size of 200 mesh and 9.5 g of pyrrole, add it to 100 mL of ethanol aqueous solution (volume ratio 1:1), stir for 10 min and mix evenly, as liquid A.
[0035] (2) Dissolve 33 g of ferric chloride and 0.1 g of polyvinyl alcohol in 100 mL of ethanol aqueous solution (volume ratio 1:1) as liquid B.
[0036] (3) Liquid A and liquid B were mixed uniformly and then transferred to a sand mill reactor, and mixture C was obtained after continuous sand mill reaction at 2000 rpm for 6 hours.
[0037] (4) The mixture C was washed several times with deionized water and filtered to obtain solid D.
[0038](5) The solid D was washed and filtered again with 300ml of ethanol, and vacuum-dried at 60° C. to obtain a graphene / polypyrrole composite electrode material. ...
Embodiment 3
[0040] In this embodiment three, the graphene / polyaniline / polypyrrole composite electrode material is prepared by mechanochemical polymerization, which specifically includes the following steps:
[0041] (1) Add 0.6g of expanded graphite, 4.3g of aniline, 4.3g of pyrrole, 2g of polyvinylpyrrolidone, and 1.0g of phytic acid into 100mL of ethanol aqueous solution (volume ratio 2:1), stir for 20min, and use it as liquid A.
[0042] (2) Take 8.6g of ferric chloride and dissolve it in 100mL of ethanol aqueous solution (volume ratio 2:1) as liquid B.
[0043] (3) After mixing liquid A and liquid B evenly, use a high-speed shear disperser to react at 25,000 rpm for 2 hours to obtain mixture C, and control the temperature to <50°C.
[0044] (4) The mixture C was repeatedly washed with deionized water and filtered to obtain solid D.
[0045] (5) The solid D was washed with ethanol, filtered, and vacuum-dried at 60° C. to obtain a graphene / polyaniline / polypyrrole composite electrode ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacitance | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com