A method for storing a tumor cell vesicle preparation
A technology of tumor cells and vesicles, which is applied in the storage field of tumor cell vesicle preparations, can solve the problems of wasting manpower and preparation material costs, which is not conducive to large-scale preparation, storage, and ready-to-use, so as to maintain biological functions, take with convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation, cryopreservation and thawing of H22 cell-derived vesicles
[0029] 1. Experimental materials and instruments
[0030] H22 mouse hepatoma cells (H22 cells), RPMI 1640 medium, 0.9% saline, methotrexate, high-speed centrifuge, ultraviolet device (conventional cell ultra-clean workbench comes with).
[0031] 2. Experimental steps
[0032] (1) Resuspend H22 mouse hepatoma cells in serum-free RPMI 1640 medium to make the cell concentration reach 1×10 7 / mL, a total of 17ml. UV irradiation was performed for 1 h, and then the chemotherapeutic drug methotrexate was added to make the drug concentration in the culture medium reach 1 mg / mL, and incubated in an incubator for 18-20 h.
[0033] (2) Gradual centrifugation to obtain vesicles derived from H22 mouse hepatoma cells:
[0034] Centrifuge at 1500 rpm for 8 min and discard the precipitate; centrifuge at 5000 rpm for 8 min and discard the precipitate; centrifuge at 14,000 g for 1 min and discard the ...
Embodiment 2
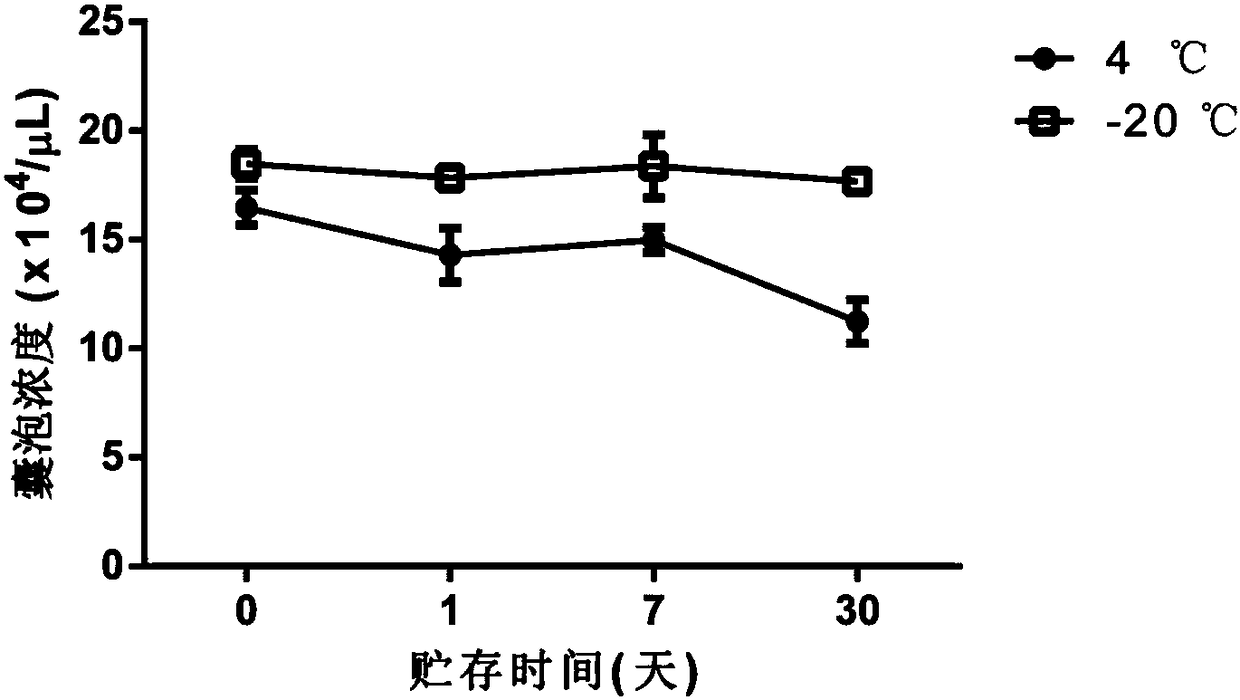
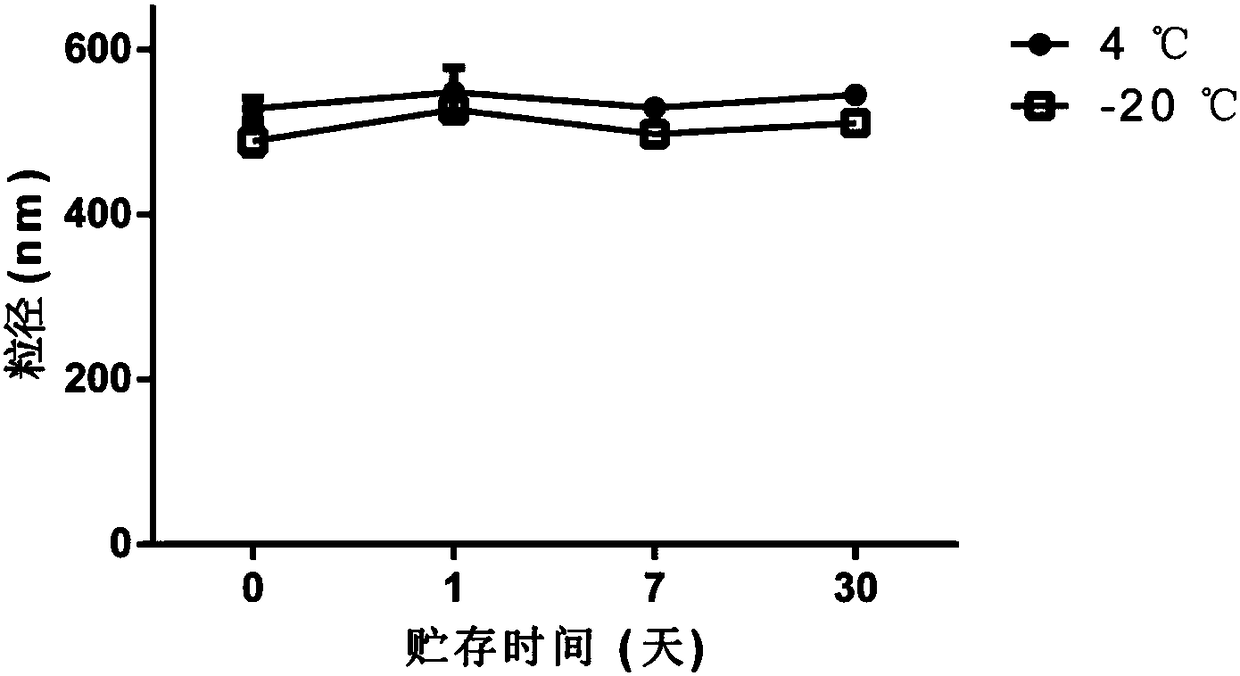
[0037] Example 2: Counting and particle size detection of H22 cell-derived vesicles
[0038] 1. Experimental materials
[0039] H22 mouse liver cancer cells, RPMI 1640 medium, 0.9% saline, methotrexate, ultraviolet device (conventional cell ultra-clean workbench comes with), high-speed centrifuge, flow cytometer, laser particle size analyzer.
[0040] 2. Experimental steps
[0041] (1) The vesicles derived from H22 cells were prepared, isolated, extracted, cryopreserved, and thawed according to the method of Example 1.
[0042] (2) Wash the flow tube, add 500 μL of ultrapure water; add 10 μL of commercial 3 μm particle size polystyrene microsphere solution and 10 μL of vesicles, shake well, and measure the vesicle concentration by flow cytometry. The calculation formula is: :
[0043] Vesicle concentration = vesicle percentage / microsphere percentage × 6.77 × 10 7 / mL
[0044] (3) Dilute H22 vesicles to 1×10 6 / mL, use a laser particle size analyzer (DLS) to detect the pa...
Embodiment 3
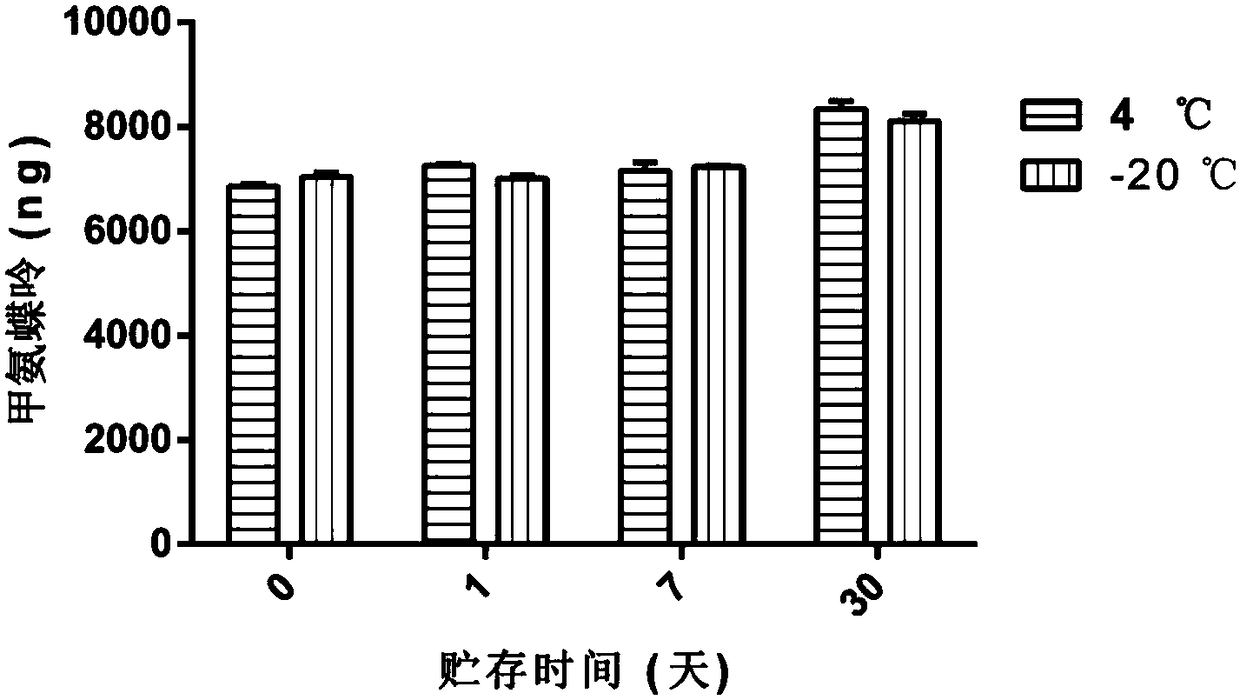
[0048]Example 3: Detection of Chemotherapy Drug (Methotrexate) Content Encapsulated in H22 Cell-derived Vesicles
[0049] 1. Experimental materials and instruments
[0050] H22 mouse liver cancer cells, RPMI 1640 medium, 0.9% saline, methotrexate, UV device (conventional cell ultra-clean workbench comes with), cell lysis solution, acetonitrile, chloroform, high-speed centrifuge, ultrasonic cell grinder ,High performance liquid chromatography.
[0051] 2. Experimental steps
[0052] (1) The vesicles derived from H22 cells were prepared, isolated, extracted, cryopreserved, and thawed according to the method of Example 1.
[0053] (2) The H22 cell-derived vesicles were centrifuged at 14,000 g for 1 h, and the supernatant was removed to leave the precipitate. Add 500 μL of cell lysate, pipette evenly, and incubate on ice for 20 min.
[0054] (3) Use an ultrasonic cell pulverizer to pulverize for 1 min to destroy the membrane structure of the vesicles to release the drug. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com