A carvanol solubilize solid composition and its preparation method and application
A carvacrol, solidification technology, applied in the field of pharmacy, can solve the problems of not significantly improving the solubility of carvacrol, easy volatilization, poor stability, etc., and achieve the effect of increasing drug loading, solubility, and high inclusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
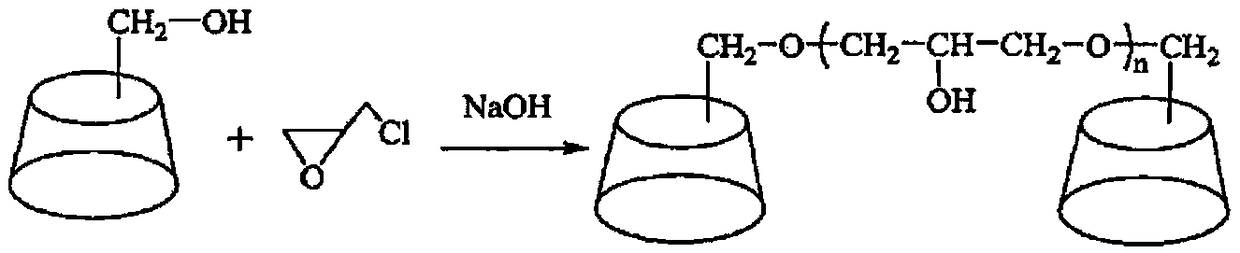
[0031] Example 1 Preparation of β-cyclodextrin polymer
[0032] Dissolve β-cyclodextrin (5.0g) in sodium hydroxide solution (10.0g) with a mass fraction of 20%, add epichlorohydrin (5.0g) at room temperature, and react for 4 hours, then add an appropriate amount of acetone to terminate the reaction , and adjust the pH to 7.0 with 1mol / L hydrochloric acid, put it into a dialysis bag with a molecular weight cut-off of 8000-12000, and after 2 days of dialysis, freeze-dry to obtain a white powder (4.86g), which is the β-cyclodextrin polymer (from infrared spectroscopy). , successful grafting).
Embodiment 2
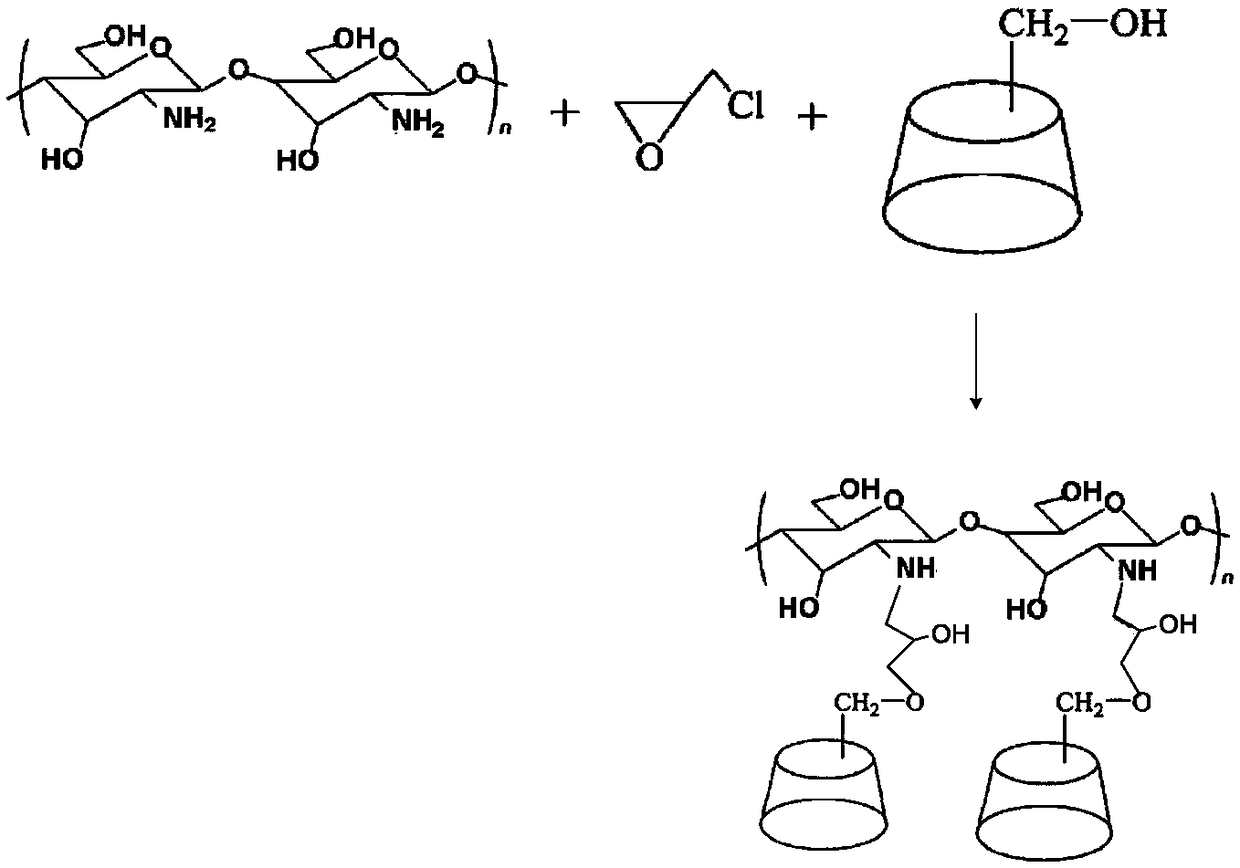
[0033] Preparation of embodiment 2 chitosan grafted β-cyclodextrin
[0034] Dissolve chitosan (2.0g) in hydrochloric acid solution (100g) with a mass fraction of 1%, add epichlorohydrin (4.0g) under stirring, heat up to 85°C, react for 5 hours, cool to room temperature, filter , Dissolve the filter cake in water (100mL), add potassium carbonate (4.0g) and β-cyclodextrin (2.0g), heat to reflux temperature and react for 3 hours, cool to room temperature, add an appropriate amount of acetone to terminate the reaction, and use Adjust the pH to 7.0 with 1mol / L hydrochloric acid, put it into a dialysis bag with a molecular weight cut-off of 8000-12000, and after 2 days of dialysis, freeze-dry to obtain chitosan-grafted β-cyclodextrin (3.76g). branch success.
Embodiment 3
[0036] (1) Dissolve the β-cyclodextrin polymer (5.0 g) prepared in Example 1 in water (15.0 g) at 50° C. to obtain a clear solution A;
[0037] (2) Mix 0.5 mL of carvacrol with 0.5 mL of absolute ethanol to form solution B;
[0038] (3) Add solution B dropwise to solution A, keep stirring at 50°C for 6 hours, cool to room temperature, place in a refrigerator at 4°C for 10 hours, filter, and precipitate in turn with water (4°C cold water), absolute ethanol (4 ℃ cold absolute ethanol) after washing, vacuum drying to obtain the carvacrol solubilized solid composition (5.10g, measured the absorbance at 295nm by ultraviolet spectrophotometer, draw inclusion rate 89.4%, carvacrol Content 8.55%, hereinafter referred to as product A).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com