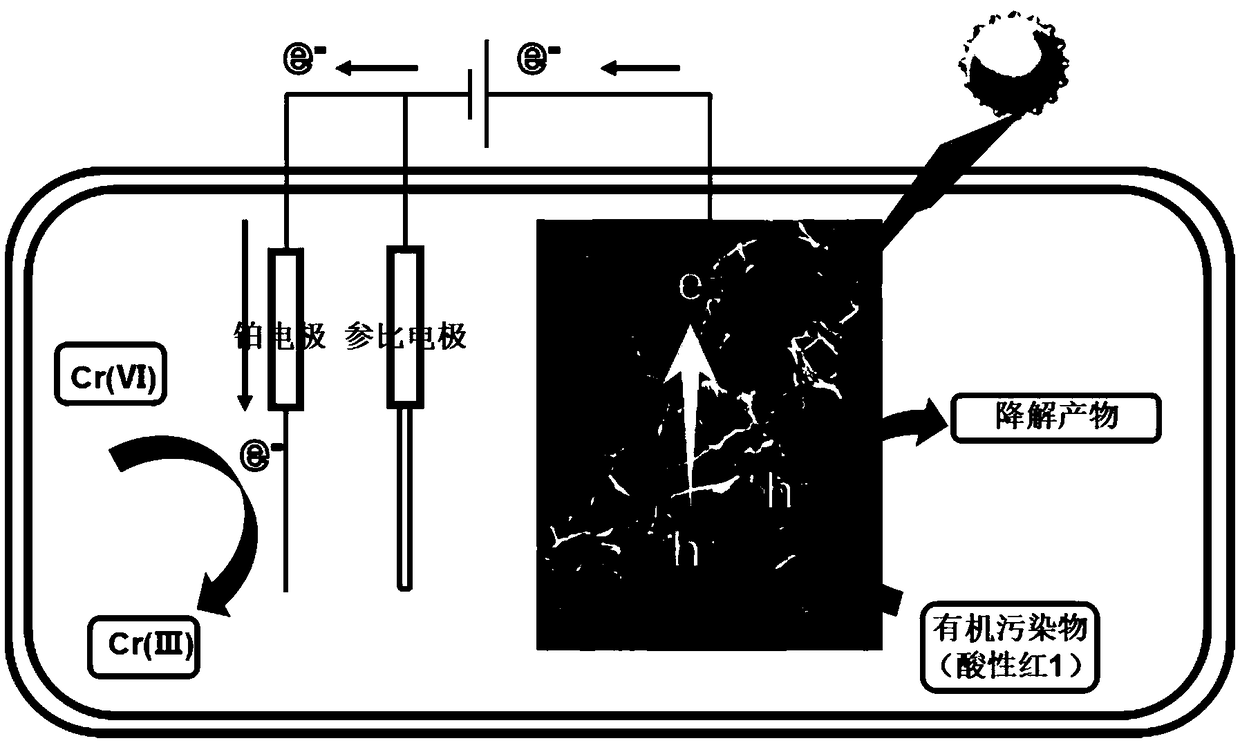
Foam nickel composite material and preparation method thereof, and application of foam nickel to photoelectrocatalytic removal of pollutants in water body
A technology of composite materials and nickel foam, which is applied in the preparation of composite materials, nickel foam composite materials and its preparation, and the application fields of photoelectric catalytic removal of pollutants in water bodies, can solve the problem of low surface adsorption rate, large band gap, difficult Recycling and other issues, to achieve the effect of solving recycling and reuse, good electrical conductivity, and narrow band gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of foamed nickel-supported zinc oxide composite material (NF@ZnO) is as follows:
[0033] Dissolve 4.958 g of zinc nitrate hexahydrate and 2.59 g of zinc formate in deionized water to make a 100 ml mixed solution, which is used as the electrolyte solution, using a three-electrode system (nickel foam cut into a size of 1 cm×2.5 cm, The surface was ultrasonically washed with dilute hydrochloric acid as the working electrode, the platinum wire electrode was used as the counter electrode, and the calomel electrode was used as the reference electrode). Electrodeposition was performed on the electrochemical workstation CHI660E, and the temperature of the electrolyte solution during electrodeposition was 25 °C. Firstly, a voltage of -1.3 V was set on the working electrode for 10 s; after that, a voltage of -1.0 V was set on the working electrode for 200 s, and repeated twice under this voltage. The samples were taken out from the working electrode and dried in ...
Embodiment 2
[0035] Dissolve 4.958 g of zinc nitrate hexahydrate and 2.59 g of zinc formate in deionized water to make a 100 ml mixed solution, which is used as the electrolyte solution, using a three-electrode system (nickel foam cut into 1 cm × 2.5 cm The size and surface were ultrasonically washed with dilute hydrochloric acid and used as the working electrode, the platinum wire electrode as the counter electrode, and the calomel electrode as the reference electrode). First, the voltage of the working electrode was set to -1.3 V for 10 s; then the voltage of the working electrode was set to -1.0 V for 200 s, and the deposition was repeated twice at this voltage. The samples were taken out from the working electrode and dried in a blast oven at 60 °C for 1.5 h. After that, it was placed in a tube furnace and calcined for 1 h at a temperature of 350 °C (heating rate: 2 °C / min) in an argon atmosphere to obtain a foamed nickel-supported zinc oxide composite material (NF@ZnO). The photocurr...
Embodiment 3
[0037] Dissolve 4.958 g of zinc nitrate hexahydrate and 2.59 g of zinc formate in deionized water, stir to make a 100ml mixed solution, use this mixed solution as the electrolyte solution, and use a three-electrode system (the treated nickel foam is used as the working electrode , a platinum wire electrode as a counter electrode, and a calomel electrode as a reference electrode), electrodeposition was performed on an electrochemical workstation CHI660E, and the temperature of the electrolyte solution during electrodeposition was 85 °C. Firstly, a voltage of -1.3 V was set on the working electrode for 10 s; after that, a voltage of -1 V was set on the working electrode for 200 s, and repeated twice under this voltage. The samples were taken out from the working electrode and dried in a forced air oven at 60 °C for 1.5 h. After that, it was placed in a tube furnace and calcined for 1 h at 350 °C (heating rate: 2-5 °C / min) in an argon atmosphere to obtain a nickel-foamed zinc oxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com