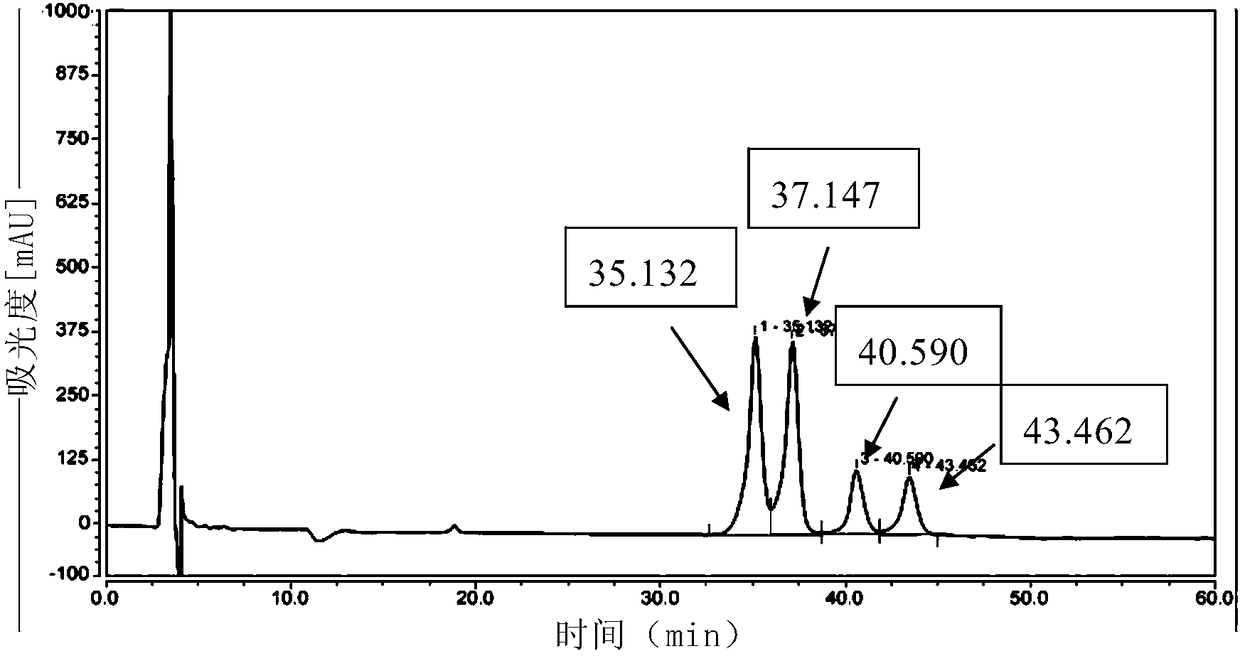
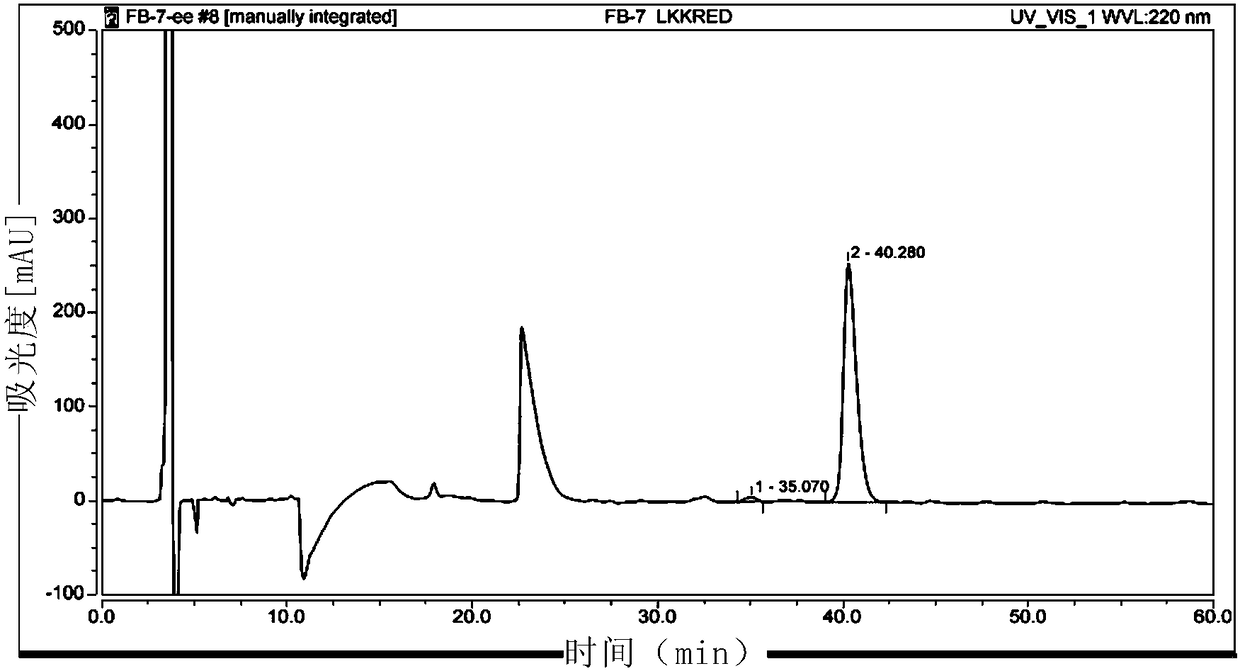
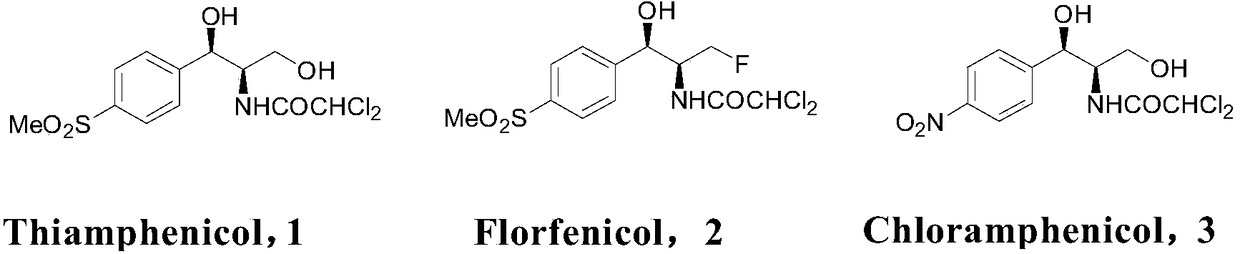
Biological preparation of key intermediate of thiamphenicol and florfenicol
A technology of intermediates and substrates, applied in the field of medicine, can solve the problems of severe environmental pressure, serious water pollution, solid waste pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0163] The preparation of the enzyme reduction substrate compound X can refer to the method described in Tetrahedron.2016, 72:1787-1793.
[0164] method
[0165] 1. Enzyme preparation method
[0166] Through conventional techniques in the field, the above-mentioned glucose dehydrogenase for coenzyme regeneration and the target gene were constructed on the same plasmid pET28a(+) vector, and then introduced into the expression host Escherichia coli, and the bacterium containing the target dual enzyme was obtained by inducing expression. Bacterial cells can be obtained directly by centrifugation, or they can be broken to obtain crude enzyme liquid, and the crude enzyme powder is used for subsequent biotransformation reactions.
[0167] 2. Method for preparing compound Y by biocatalytic reduction of compound X
[0168] The invention provides a method for preparing compound Y by catalyzing reduction of compound X by carbonyl reductase. The reaction formula is as follows:
[016...
Embodiment 1
[0179] Embodiment 1, the construction of carbonyl reductase engineering bacteria
[0180] The EA carbonyl reductase target gene and the glucose dehydrogenase target gene were entrusted to a commercial company to carry out the whole gene synthesis, cloned into the pET28a(+) vector, transformed into Escherichia coli DH5α competent cells, cultured on the plate, and picked a single colony of positive transformants After extracting and sequencing the plasmid, the recombinant plasmid was extracted, introduced into BL21 (DE3) strain, cultured in LB, and genetically engineered bacteria capable of inducing the expression of recombinant carbonyl reductase and alcohol dehydrogenase were obtained.
Embodiment 2
[0181] Embodiment 2, the preparation of recombinant carbonyl reductase, glucose dehydrogenase
[0182] Inoculate the genetically engineered bacterium stored in glycerol in the previous step into the LB liquid medium containing kanamycin, cultivate for 13 hours at 37°C and 220rpm to obtain the seed medium, and inoculate the seed culture medium in a proportion of 1.5%. On the liquid medium containing 50ug / ml kanamycin resistance, then cultivate to OD at 37℃, 220rmp 600 If the value is >2.0, add lactose with a final concentration of 1.0%, cool down to 25°C, continue to cultivate for 3 hours, add lactose with a final concentration of 0.5%, and cultivate for 20 hours, put it in a tank, and centrifuge to obtain bacteria, which is ready for biotransformation.
[0183] The fermentation formula is as follows:
[0184] raw material
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com