Application of oleanolic acid in preparation of drugs for treatment of autoimmune diseases
An autoimmune disease, oleanolic acid technology, applied in the field of biomedicine, can solve problems such as uncertainty, and achieve the effect of obviously inhibiting function, inhibiting secretion, and reducing the degree of kidney damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
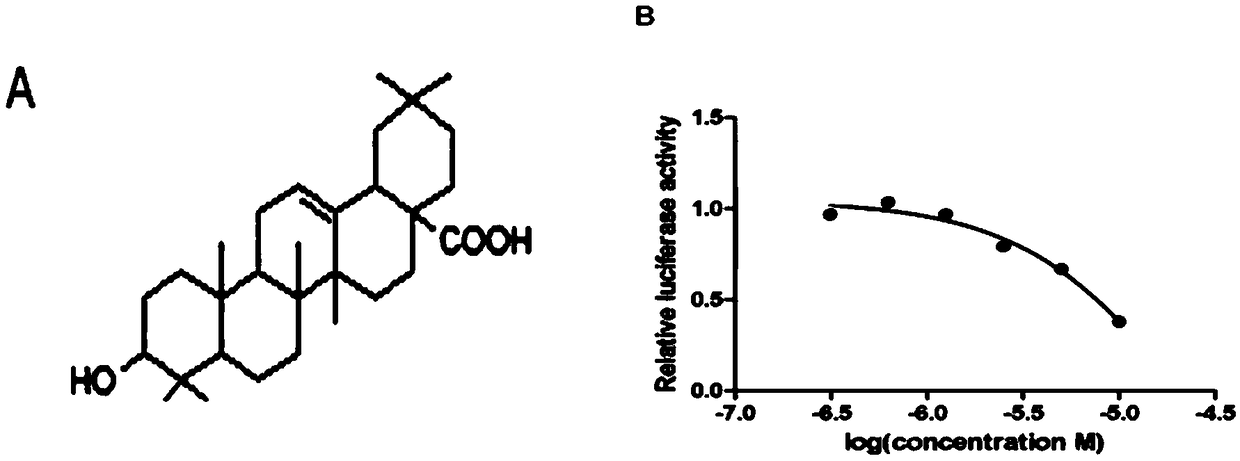
[0021] Example 1 The EC of oleanolic acid in RORγt--Jurkat cells 50 test
[0022] 1. The luciferase activity screening system based on transcription factor activity is constructed as follows:
[0023] Material:
[0024] The cell line Jurkat was preserved in our laboratory; the DH5α bacterial strain was donated by Professor Ma Runlin, Institute of Genetics, Chinese Academy of Sciences, Beijing; the restriction endonuclease was purchased from Fermentas Company of the United States; the DNA ligase was purchased from NEB Company of the United States; the reporter gene sequence IRES-GFP was invented Preserved by people themselves (other existing reporter gene sequences can also be used); reporter plasmid pGL4.31[luc2P / GAL4UAS / Hygro] plasmid and pBIND plasmid were purchased from Promega Company in the United States; DMEM medium, RPMI1640 medium and cultured cells were used Sodium pyruvate, glutamine, β-mercaptoethanol, non-essential amino acids, and double antibodies were purchase...
Embodiment 2
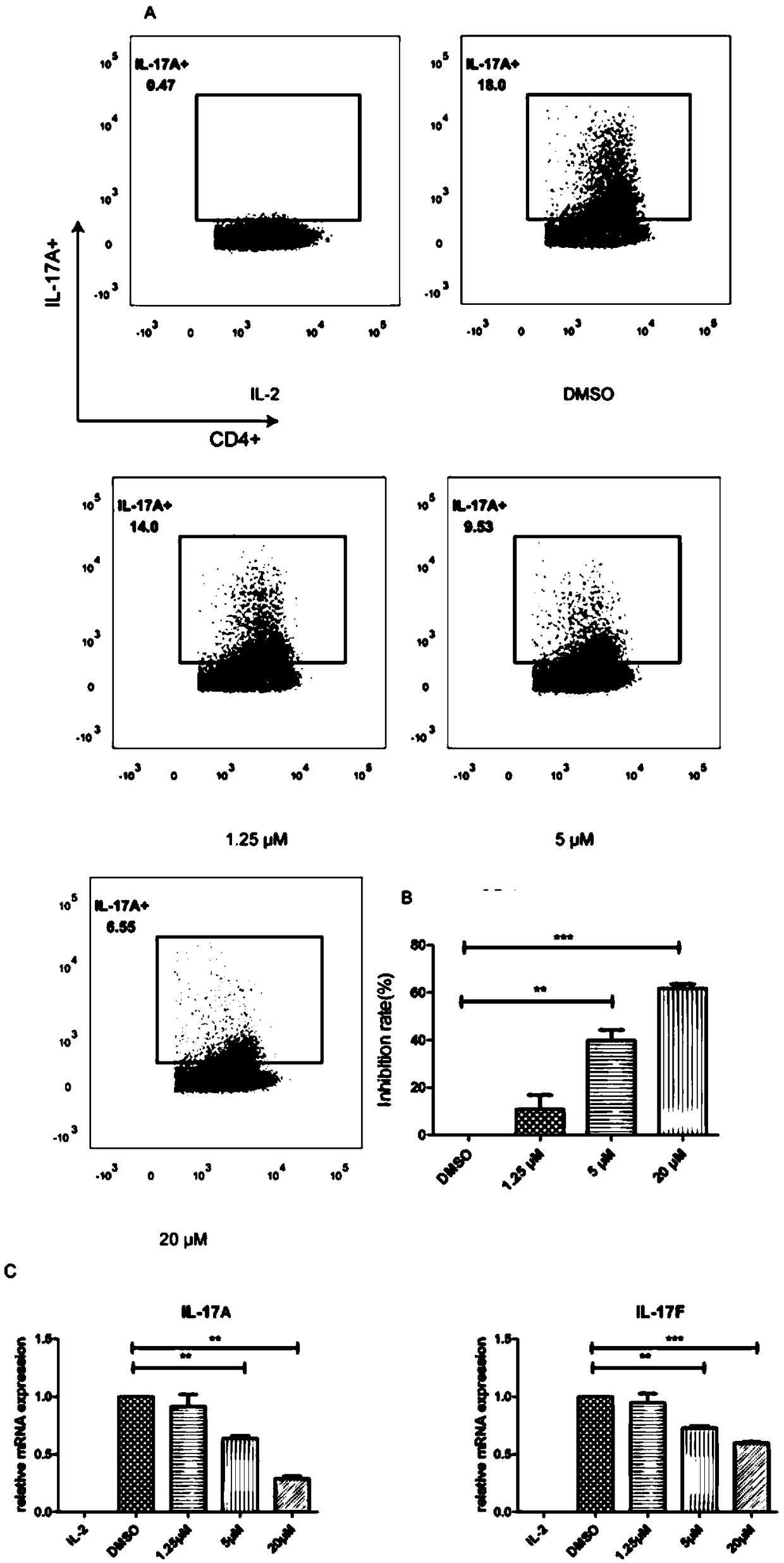
[0033] Example 2 Test of oleanolic acid's inhibitory effect on Th17 cells, IL-17A, IL-17F
[0034] Through in vitro Th17 cell differentiation experiments, it was verified that oleanolic acid has the function of inhibiting Th17 differentiation. The inhibitory effect of oleanolic acid on Th17 cells, IL-17A, and IL-17F was studied by flow cytometry detection, and the detection results were as follows: figure 2 A. figure 2 B. figure 2 C shown.
[0035] Experimental operation: The night before the experiment, a 12-well plate was coated with a PBS solution containing 5 μg / mL CD3 antibody and 1 μg / mL CD28 antibody, 0.5 mL per well, and coated overnight at 4°C. The experiment was carried out on the second day. First, the CD4+ T cells in the mouse spleen were sorted with Miltenyi magnetic beads, and the sorted cells were divided into 1×10 6 Resuspend the cells at a cell density of 1 / mL, add 1 mL of cell suspension to each well of the coated 12-well plate, and add hTGF-β at a fin...
Embodiment 3
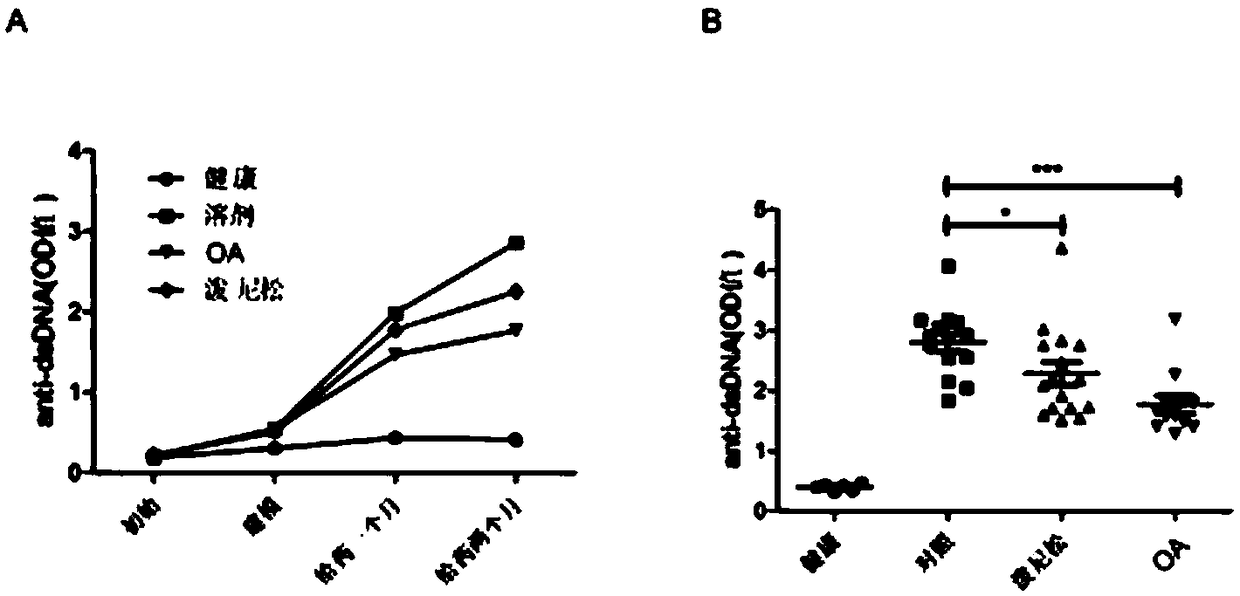
[0040] Example 3 Oleanolic acid inhibits serum anti-dsDNA antibodies in a mouse model of lupus nephritis and the therapeutic effect of nephritis
[0041] To establish the lupus nephritis model induced by pristane, a single injection of 500 μL pristane was administered intraperitoneally, and a single injection of 500 μL normal saline was used as the control. Oleanolic acid was compared with the solvent control and the positive drug prednisone. The drugs were all dissolved in 25% dehydrated alcohol and 75% hydroxypropyl beta-cyclodextrin (30% content), and 100 μL was administered intragastrically. Administered twice a week for 2 consecutive months. Oleanolic acid was compared with solvent control and positive drug prednisone to study the inhibitory effect of oleanolic acid on anti-dsDNA antibody in mouse serum.
[0042] Operation of ELISA detection of anti-dsDNA antibodies in serum: (1) Pretreatment plate: poly-lys (2 μg / mL), 100 μL / well, incubate at 37°C for 1 h; wash plate: w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com