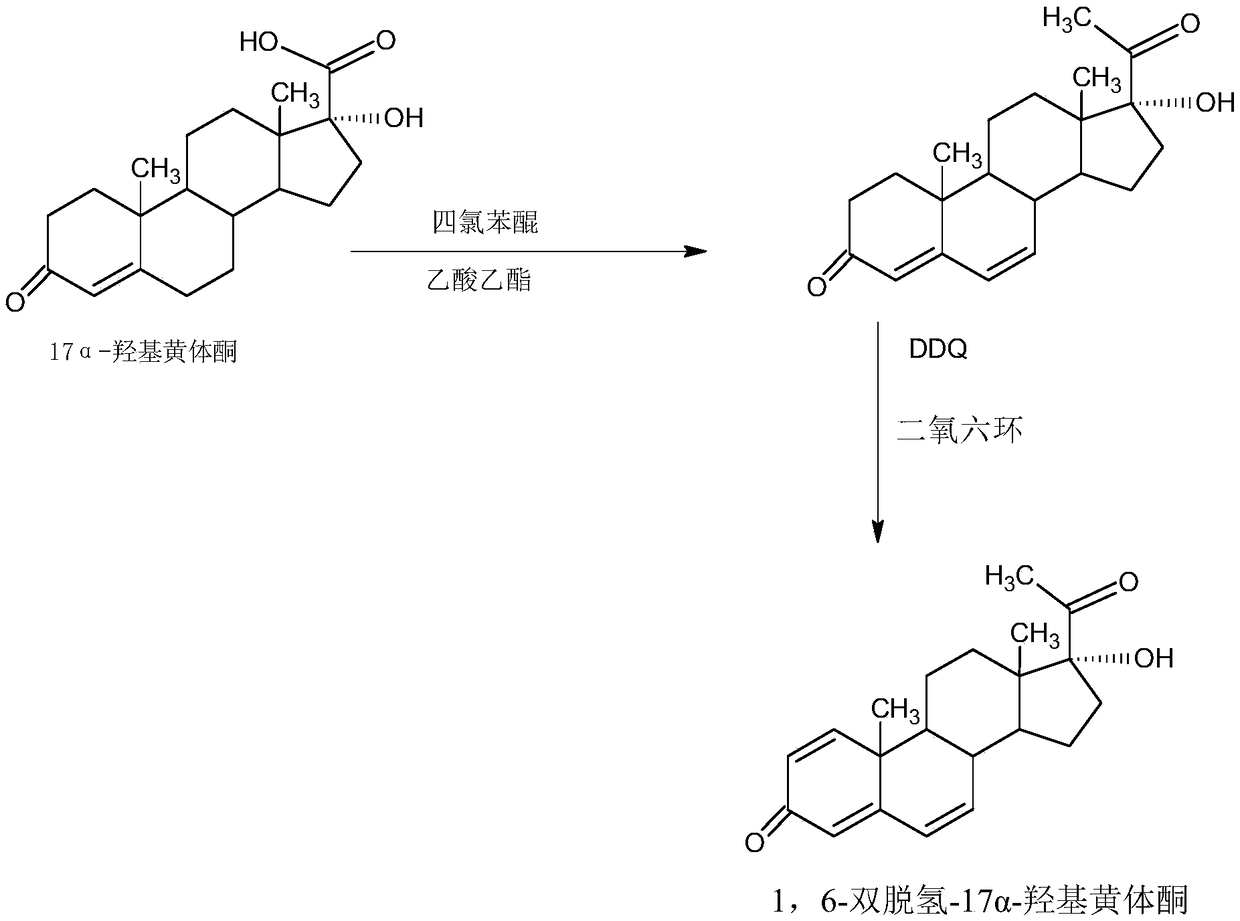
Method for preparing 1,6-didehydro-17 alpha-hydroxyprogesterone
A technology of hydroxyprogesterone and double dehydrogenation, applied in the direction of steroids, organic chemistry, etc., can solve the problems of DDQ dehydrogenation agent, etc., and achieve the effect of high product yield, low production cost and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
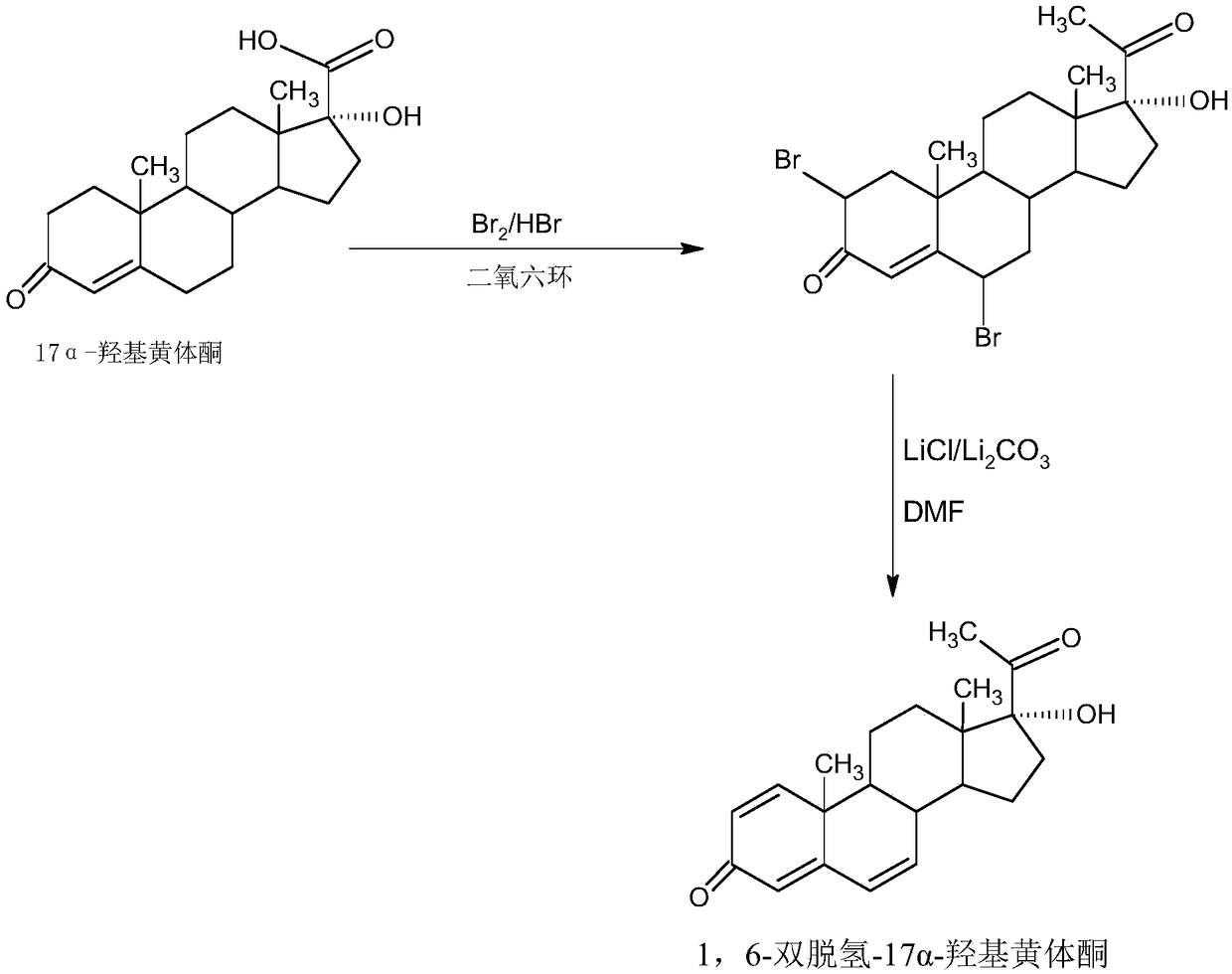
[0023] B. Preparation of debrominated compounds
[0024] Dissolve the above bis-bromide in an organic solvent, add lithium chloride and lithium carbonate, heat up to 40-80°C and stir to dissolve, then keep warm at 40-120°C for 2-6 hours, TLC confirms the reaction end point, after the reaction, Adjust the pH to neutral, concentrate under reduced pressure to recover the organic solvent, then cool; add tap water, stir and crystallize at 5-25°C for 3-6 hours, centrifuge, wash, and dry to obtain the debrominated product: 1,6-didehydro- 17a-hydroxyprogesterone crude product, HLPC content 97.0-98.5%, weight yield 62.5.-65.0%.
[0025] C. Crude refined
[0026] Decolorize and recrystallize the above-mentioned crude debrominated product with activated carbon in low-carbon alcohols below C4 to obtain 1,6-didehydro-17a-hydroxyprogesterone product, the HPLC content is 99.0-99.5%, and the melting point is 228-232°C. This step The weight yield of the synthesis reaction is 50-55%.
[0027...
Embodiment 1
[0035] The preparation of step A, two bromines
[0036] In a 2000ml three-necked flask, add 100g 17a hydroxyprogesterone, 200ml dioxane, 80g 25% hydrobromic acid acetic acid solution, stir to make the system strongly acidic, control the temperature at 25-30°C, and slowly add 140g The solution made of bromine and 600ml dioxane should be dripped within about 1.0-1.5 hours. After the dripping, continue to keep warm at 25-30°C for 4-6 hours. TLC confirms the reaction end point. After the reaction, slowly Slowly add 200ml of 30% hydrosulfite aqueous solution to completely destroy bromine, then concentrate under reduced pressure to recover 90-95% of dioxane, after concentration, cool down to 10-15°C, add 600ml of tap water, stir and crystallize Centrifuge for 3-4 hours, wash with water until neutral, and dry under vacuum below 40°C to obtain 144.8g of bisbromide 2,6-dibromo-17a-hydroxyprogesterone, HLPC content 98.2%, moisture content 3.5%, weight yield 144.8%;
[0037] Step B, synt...
Embodiment 2
[0042] The preparation of step A, two bromines
[0043] In a 2000ml three-necked flask, add 100g 17a hydroxyprogesterone, 500ml toluene, 80g 25% hydrobromic acid aqueous solution, stir to make the system strongly acidic, control the temperature at 25-30°C, slowly add 140g bromine and 300ml The solution made of DMF will be dropped within about 1.0-1.5 hours. After the drop, continue to keep warm at 25-30°C for 4-6 hours. TLC will confirm the reaction end point. After the reaction, slowly add 200ml of 30% Sodium hydrosulfite aqueous solution to completely destroy bromine, then wash twice with 600ml tap water, separate the water, dry with 50g anhydrous magnesium sulfate, and concentrate under reduced pressure to recover 90-95% of the mixed solvent of toluene and DMF. After concentration, Cool down to 10-15°C, add 600ml of tap water, stir and crystallize for 3-4 hours, centrifuge, wash with water until neutral, and dry in vacuum below 40°C to obtain the dibromo 2,6-dibromo-17a-hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com