An AG-MOFS metal organic frame material, synthesis method and its application in ion recognition
A technology of metal-organic frameworks and anions, applied in silver-organic compounds, luminescent materials, analytical materials, etc., can solve the instability of MOFs and other problems, and achieve the effects of easy control of the reaction, mild synthesis conditions, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
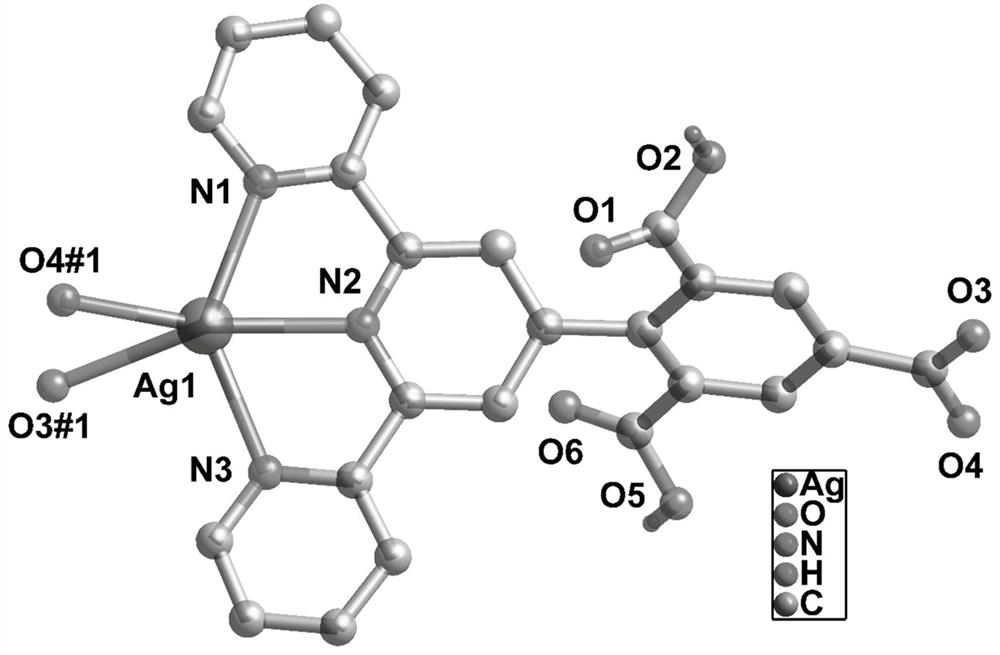
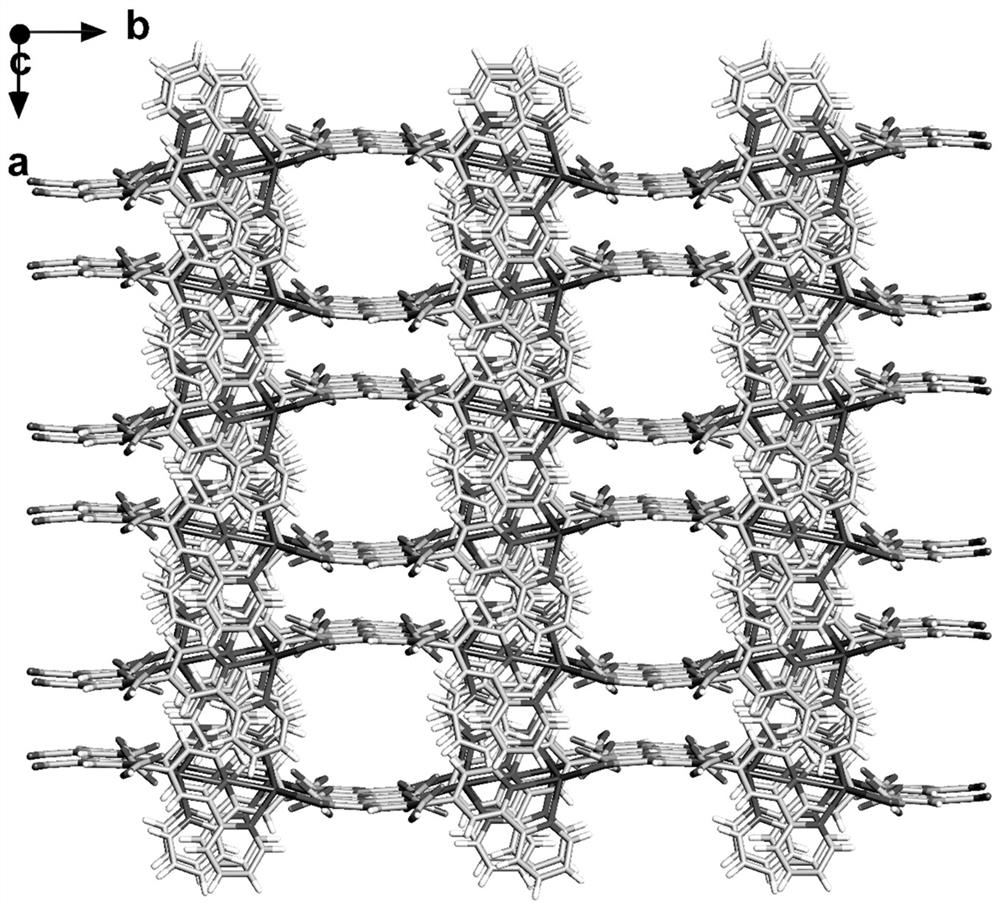
[0042] Take 0.15mmol of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine, 0.05mmol of silver nitrate, 6ml of high-purity water, and 6ml of acetonitrile; In a lined stainless steel reaction kettle, add a dilute nitric acid solution with a concentration of 0.5mM dropwise, adjust the pH value of the reaction system to 5, and conduct a constant temperature solvothermal reaction at 120°C for 80 hours to obtain yellow blocky crystals, namely 4-(2,4,6 -Carboxyphenyl)-2,2':6',2"-terpyridine Ag metal organic framework material.
[0043] Add 5 mg of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine Ag metal-organic framework to 10 mL of M(NO 3 ) x In aqueous solution (M=Al 3+ ,Fe 2+ , Na + , K + ,Zn 2+ ,Fe 3+ ,Cd 2+ ,Pb 2+ ,Cu 2+ ,Mg 2+ ,Mn 2+ ,Co 2+ ,In 3+ , Ni 2+ ,Hg 2+ ), ultrasonically oscillate at room temperature for 30 minutes, take it out and put it into a 4ml cuvette, and test the fluorescence intensity on a fluorescence photometer. Such as Figure 4 As shown, Fe c...
Embodiment 2
[0045] Take 0.15mmol of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine, 0.05mmol of silver nitrate, 6ml of high-purity water, and 6ml of acetonitrile; In a lined stainless steel reaction kettle, add a dilute nitric acid solution with a concentration of 0.5mM dropwise, adjust the pH value of the reaction system to 5, and react at a constant temperature of 120°C for 80h to obtain yellow blocky crystals, that is, 4-(2,4,6-carboxy Phenyl)-2,2':6',2"-terpyridine Ag metal-organic framework materials.
[0046] Add 5 mg of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine Ag metal-organic framework material to 10 mL containing different anions (Cl - , SO 4 2- ,SCN - , SO 3 2- ,NO 3 - ,I - ,ClO 4 - ,CO 3 2- ,Br - , F - , Ac - ,H 2 PO 4 - ,MnO 4 2- ,CrO 4 2- ,Cr 2 o 7 2- ) aqueous solution, ultrasonically oscillate at room temperature for 30 minutes, take it out and put it into a 4ml cuvette, and test the fluorescence intensity on a fluorescence photometer. Such a...
Embodiment 3
[0048] Take 0.15mmol of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine, 0.05mmol of silver nitrate, 6ml of high-purity water, and 6ml of acetonitrile; In a lined stainless steel reaction kettle, add a dilute nitric acid solution with a concentration of 0.5mM dropwise, adjust the pH value of the reaction system to 5, and react at a constant temperature of 120°C for 80h to obtain yellow blocky crystals, that is, 4-(2,4,6-carboxy Phenyl)-2,2':6',2"-terpyridine Ag metal-organic framework material.
[0049] Add 5 mg of 4-(2,4,6-carboxyphenyl)-2,2':6',2"-terpyridine Ag metal-organic framework to 10 mL of Fe 3+ In the aqueous solution, take it out after ultrasonic vibration at room temperature for 30 minutes, put it into a 4ml four-sided transparent cuvette, and measure the fluorescence intensity on a fluorescence photometer. Such as Image 6 , 8 As shown, it is obtained that the material has different concentrations of Fe 3+ Fluorescence quenching and fluorescence emission.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com