Method for recovering lithium and producing high-purity boric acid or borax
A high-purity lithium recovery technology, applied in borates, boron oxyacids, chemical instruments and methods, etc., can solve the problems of high acid-base consumption, organic solvent pollution of brine, and large acid consumption, etc., to achieve The effect of high lithium ion yield, wide application range and low acid and alkali consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
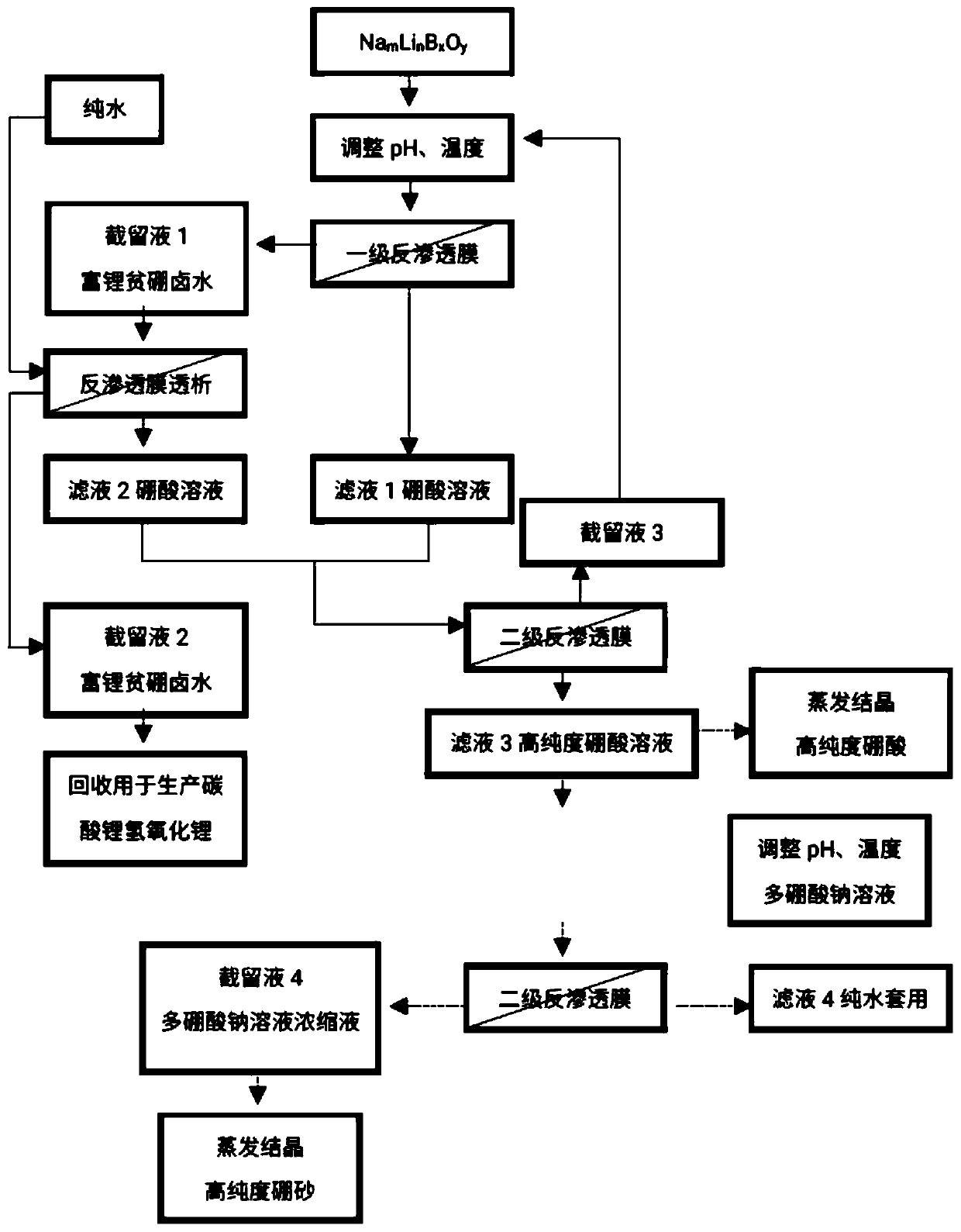
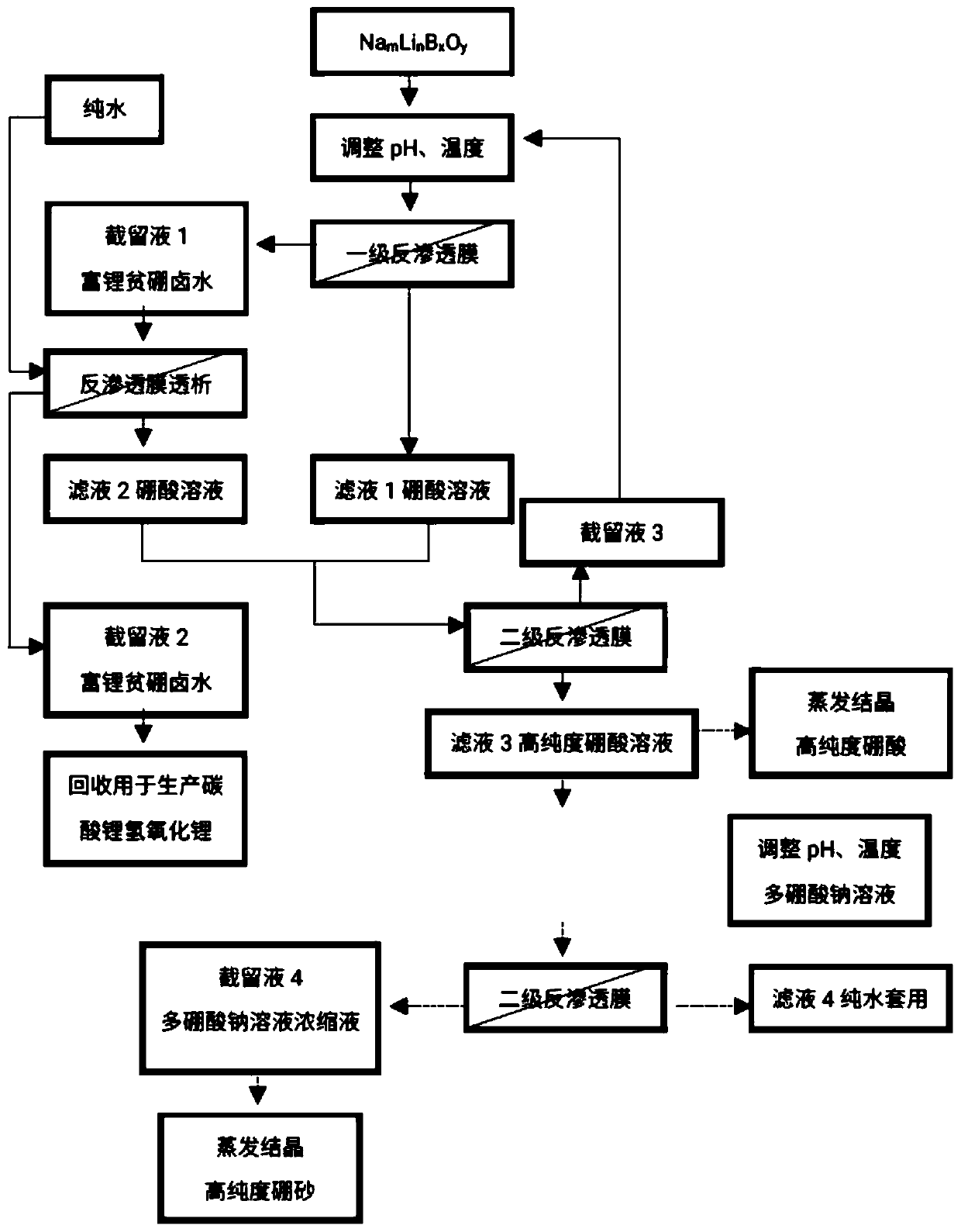
[0040] see figure 1 , the present invention provides a reverse osmosis method from Na m Li n B x o y The method for reclaiming lithium in the solution and producing high-purity boric acid or high-purity borax comprises the steps:
[0041] a. Na m Li n B x o y Add acid to the solution to adjust the pH to 2.0-7.0;
[0042] b. Pump the pH-adjusted solution into the membrane system, and apply pressure on both sides of the reverse osmosis membrane to form a pressure difference. Part of the water and H in the solution 3 BO 3 Transmembrane migration from the high pressure side to the low pressure side;
[0043] c. Lithium ions on the high-voltage side are enriched to obtain lithium-rich, boron-poor brine;
[0044] d. The aqueous solution on the low-pressure side is a boric acid solution containing a small amount of lithium ions;
[0045] Among them, the Na m Li n B x o y In the solution, the content of B element is 100-15000ppm, and the content of lithium ion is 30-10...
Embodiment 2
[0057] Preferably, there is also provided a m Li n B x o y A method for recovering lithium in solution and producing high-purity boric acid or high-purity borax. The specific steps of the method are as follows:
[0058] a) adding acid to the feed brine to adjust the pH to 2-7;
[0059] b) will adjust the pH of the Na m Li n B x o y The solution is pumped into the first-stage reverse osmosis system, and pressure is applied on both sides of the reverse osmosis membrane to form a pressure difference to obtain the retentate 1 lithium-rich boron-poor brine and the filtrate 1 boric acid solution;
[0060] c) dialyzing the retentate 1 lithium-rich and boron-poor brine with pure water to obtain the retentate 2 lithium-rich and boron-poor brine and the filtrate 2 boric acid solution;
[0061] d) performing secondary filtration on filtrate 1 and filtrate 2 boric acid solution using reverse osmosis membrane to obtain retentate 3 and filtrate 3 high-purity boric acid solution;
...
Embodiment 3
[0072] Preferably, a kind of Na is also provided m Li n B x o y In the solution, the lithium ion content is 3000ppm, the sodium ion content is 2000ppm, the boron content is 6000ppm, the pH of the brine is 10.0, and the temperature of the brine is 35°C.
[0073] Preferably, the method for reclaiming lithium and producing high-purity boric acid or high-purity borax comprises the following steps:
[0074] a) adding hydrochloric acid to the feed brine to adjust the pH to 4.0;
[0075] g) will adjust the pH of the Na m Li n B x o y The solution is pumped into the first-stage reverse osmosis system, and pressure is applied on both sides of the reverse osmosis membrane to form a pressure difference. The pressure difference on both sides of the membrane is controlled to 60 bar, and the temperature of the brine is controlled to 27°C; the intercepted solution 1 is rich in lithium and poor in boron. Brine and filtrate 1 boric acid solution;
[0076] h) Lithium ion content 6000pp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com