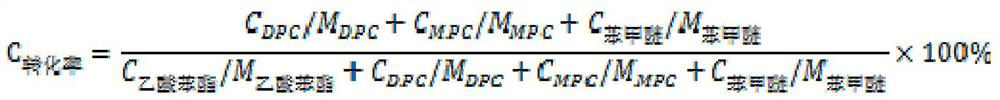The preparation method of diphenyl carbonate compound
A technology of diphenyl carbonate and diester carbonate, which is applied in the field of preparation of diphenyl carbonate compounds, and can solve the problems of low selectivity, low utilization rate of raw materials, and non-environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
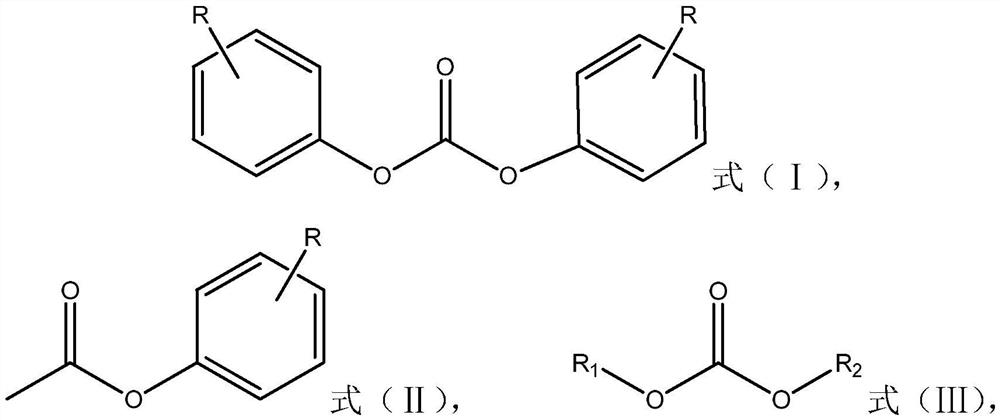
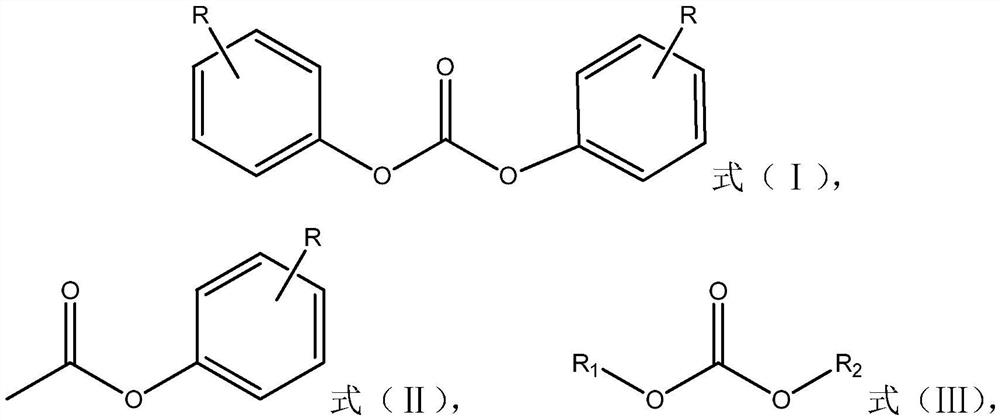
[0018] The present invention provides a method for preparing diphenyl carbonate compounds represented by formula (I), wherein the preparation method comprises: in the presence of a catalyst, making phenyl acetate compounds represented by formula (II) and The carbonic acid diester compound shown in (Ⅲ) is transesterified, and the catalyst contains 1,4-diazabicyclo[2.2.2]octane;
[0019]
[0020] where R is hydrogen or C 1 -C 4 the alkyl group, R 1 and R 2 are each independently methyl or ethyl.
[0021] Preferably, the catalyst is 1,4-diazabicyclo[2.2.2]octane.
[0022] In the present invention, preferably, R is hydrogen, methyl or ethyl, more preferably hydrogen or methyl, even more preferably hydrogen.
[0023] In the present invention, preferably, R in formula (II) is at the para-position of the ester group.
[0024] Specific examples of the phenyl acetate compounds represented by the above formula (II) include: phenyl acetate, p-cresol acetate, p-ethylphenol acetat...
Embodiment approach
[0041] According to a preferred embodiment of the present invention, the preparation method comprises: first mixing the phenyl acetate compound shown in formula (II) with the catalyst, and heating the obtained mixture to the temperature required for the transesterification reaction , and then the carbonic acid diester compound represented by formula (III) is mixed with the mixture.
[0042] According to another preferred embodiment of the present invention, the preparation method comprises: mixing the catalyst, the phenyl acetate compound shown in formula (II) and the carbonic acid diester compound shown in formula (III), to obtain The mixture is heated to the temperature required for the transesterification reaction, and after 2-5 hours of reaction, an entrainer is added to the reaction solution.
[0043] In the present invention, in order to improve the conversion rate of reactants and product selectivity, preferably, the entrainer is added to the reaction solution in 2-3 ti...
Embodiment 1
[0070] Under the protection of nitrogen, mix 20.42g of phenyl acetate and 3.36g of 1,4-diazabicyclo[2.2.2]octane in a three-necked round bottom flask equipped with a separator, and the temperature of the oil bath reaches 80°C After that, add 6.76g dimethyl carbonate (the molar ratio of phenyl acetate, 1,4-diazabicyclo[2.2.2]octane and dimethyl carbonate is 1:0.2:0.5), under normal pressure (0.1 MPa) conditions, the methyl acetate and its azeotrope generated by the simple distillation method are steamed to the liquid separator and removed while reacting. After reacting for 2 hours, cool for about 6 minutes, add entrainer toluene to the round bottom flask, add 2 times in total, the time interval between two adjacent additions is 3 hours, and add 5mL toluene each time. The reaction ended after a total of 9 hours, and the catalyst was recovered by rectification. The reaction liquid is carried out to gas chromatographic analysis, phenyl acetate conversion rate 77.1%, the selectivi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com