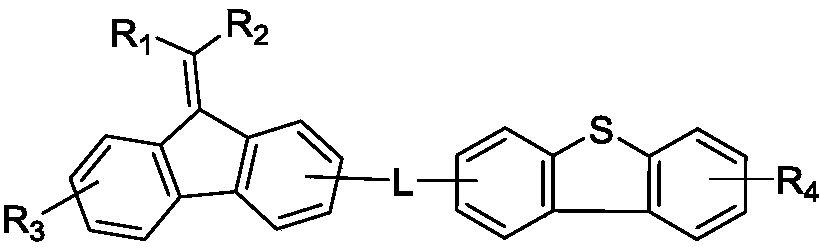
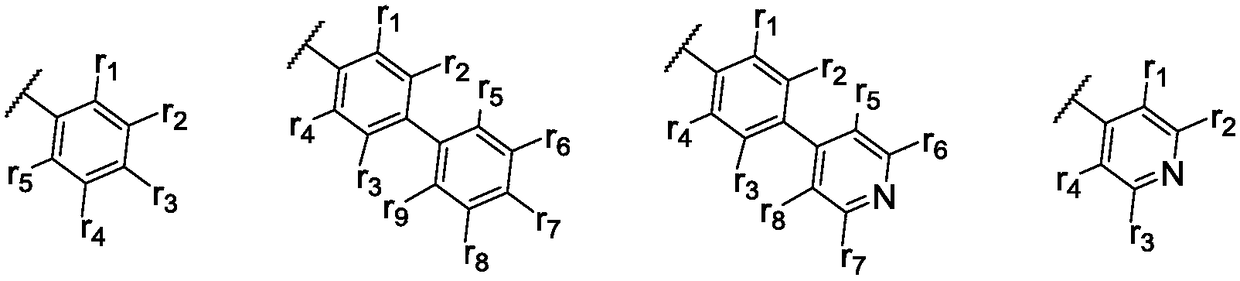
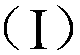Organic luminous compound and organic electroluminescence device thereof
A technology for electroluminescent devices and light-emitting compounds, which is applied in organic chemistry, electro-solid devices, electrical components, etc., to achieve the effect of high luminous efficiency and low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation method of the organic electroluminescent compound of the present invention can be prepared by a conventional coupling reaction, for example, it can be prepared by the following synthetic route, but the present invention is not limited thereto:
[0064]
[0065] In the present invention, there is no special limitation on the reaction conditions of the above reactions, and the reaction conditions well known to those skilled in the art can be adopted. The present invention has no special limitation on the sources of the raw materials used in the above reactions, which can be commercially available products or prepared by methods well known to those skilled in the art. The organic electroluminescence compound of the invention has few synthesis steps, simple treatment and easy industrial production.
[0066] The present invention also provides an organic electroluminescent device. The organic electroluminescent device is an organic electroluminescent devic...
Embodiment 1
[0071] Embodiment 1: the preparation of compound 1
[0072]
[0073] Compounds 1-1 (5.16 g, 20 mmol), 1-2 (4.14 g, 20 mmol) and sodium ethoxide were completely dissolved in 100 ml of ethanol in a round bottom flask, and the resulting solution was heated and stirred. After the reaction was completed, the residue obtained by concentrating the obtained product under reduced pressure was diluted with tetrahydrofuran, and washed with water and brine. The organic solvent layer was collected, water was removed over anhydrous magnesium sulfate, and the residue was filtered, then concentrated under reduced pressure. The concentrated solution was purified by silica gel column chromatography to prepare compound 6.70 g of compound 1-3, yield 75%.
[0074] Under the protection of nitrogen, 1-3 (44.70g, 100mmol), 1-4 (38.62g, 100mmol), potassium carbonate (1.24g, 9.00mmol), and toluene 200mL were added into a 2L reactor and stirred. The temperature in the reactor was raised to 70°C, an...
Embodiment 2
[0075] Embodiment 2: the preparation of compound 2
[0076]
[0077] Compound 2 was obtained by replacing 1-4 in Example 1 with 2-4 as shown above. Mass Spectrum m / z: 627.19 (calculated: 627.11). Theoretical element content (%)C 39 h 18 f 5 NS: C, 74.63; H, 2.89; F, 15.13; N, 2.23; S, 5.11 Measured element content (%): C, 74.70; H, 2.90; F, 15.13; N, 2.22; It was confirmed above that the obtained product was the target product 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com