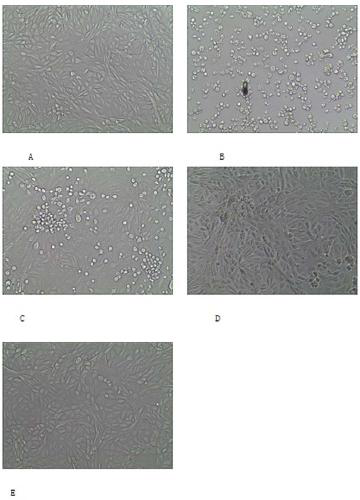
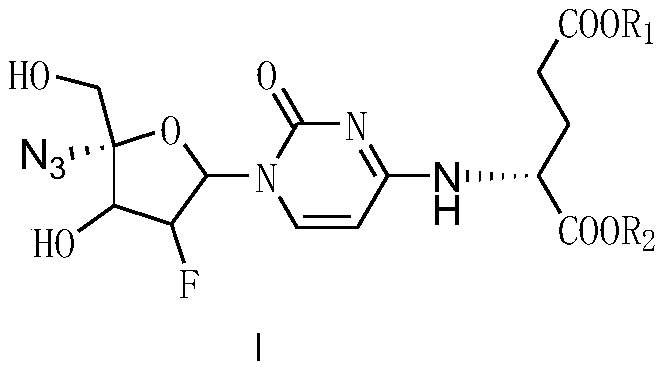
4-amino-acid-substituted pyrimidine nucleoside compound and medicinal application thereof
A technology of pyrimidine nucleoside derivatives and amino acids, which is applied in the field of pyrimidine nucleoside compounds and 4-amino acid substituted pyrimidine nucleoside compounds, and can solve problems such as 4-amino acid substituted pyrimidine nucleoside compounds that have not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
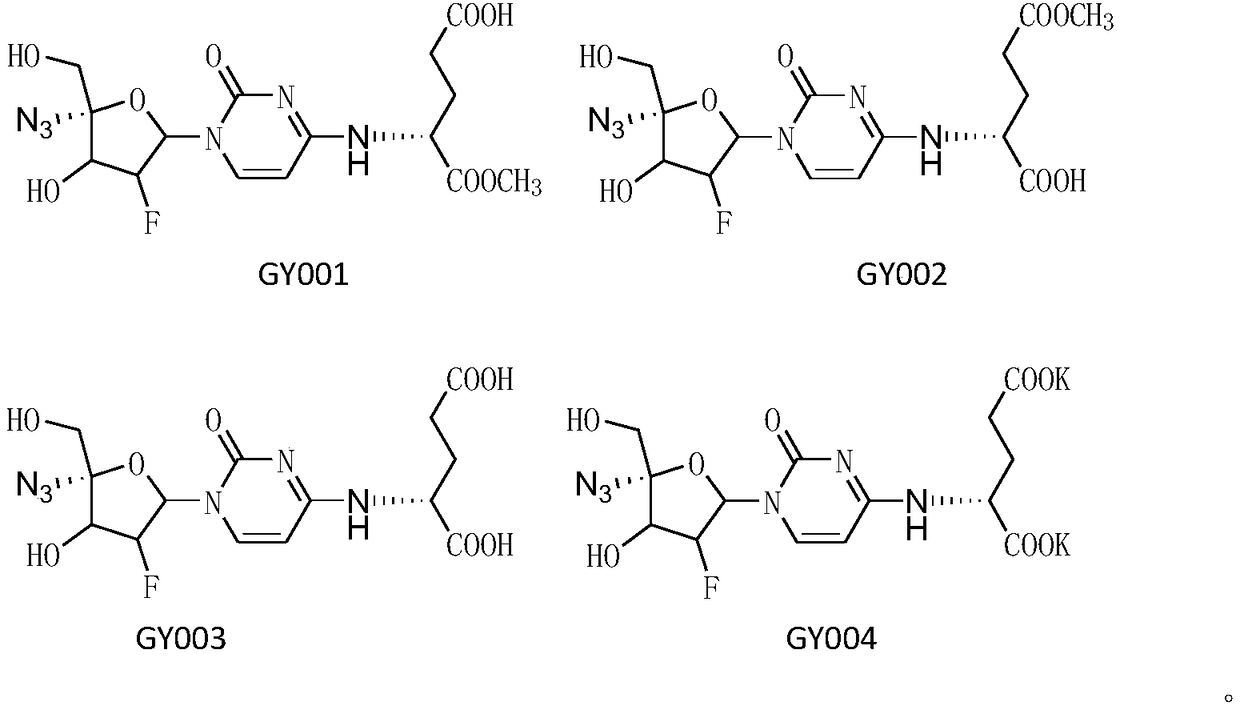
[0033] Take compound GY005 (0.44g, 1mmol), add methanol (20ml) to dissolve at room temperature, add water (20ml) and potassium carbonate (1.38g, 10mmol), and stir overnight at room temperature, the raw materials are completely reacted on the plate, and dilute hydrochloric acid (1N, 25mL), the solvent was distilled off under reduced pressure, separated by column chromatography (dichloromethane:methanol:acetic acid=90:10:1) to obtain compound GY001 (100mg, 24%) and compound GY002 (260mg, 60%), GY001: HRMS (M+Na):453.1130; 1 H-NMR (DMSO-d6, 400Hz), δ: 8.52 (brs, 1H, NH), 7.61 (d, 1H, J=8.0Hz, CH), 6.36-6.46 (m, 2H, CH 2 ), 5.93 (d, 1H, J=7.2Hz, CH), 5.74 (brs, 1H, OH), 5.10-5.24 (m, 1H, CH), 4.42-4.55 (m, 2H, CH 2 ), 3.72(s, 2H), 3.65(s, 3H, OCH 3 ), 2.28-2.33 (m, 2H, CH 2 ), 1.82-2.06 (m, 2H, CH 2 ).
[0034] GY002: HRMS (M+Na) 453.1123; 1 H-NMR (DMSO-d6, 400Hz), δ: 7.8 (brs, 1H, NH), 7.51 (d, 1H, J=8.0Hz, CH), 6.36-6.43 (m, 2H, CH 2 ), 6.0 (d, 1H, J=8.0Hz, CH), 5.71 (brs...
Embodiment 2
[0036] Take compound GY005 (0.44g, 1mmol), add methanol (20mL) to dissolve at room temperature, add sodium hydroxide solution (1M, 5mL), react at room temperature overnight, add acetic acid (0.5mL), column chromatography (dichloromethane: methanol : acetic acid=80:18:2), to obtain GY003 (415 mg, 98%) as a white powdery solid. HRMS(M+Na)439.0970: 1 H-NMR (CD 3 OD, 400Hz), δ: 7.71(d, 1H, J=8.0Hz, CH), 6.46-6.50(dd, 1H, J=4.8, 7.2Hz, CH), 6.1(d, 1H, J=8.0Hz, CH), 5.11-5.27 (ddd, 1H, J=4.0, 4.2, 44.4Hz, CH), 4.83 (brs, 1H, CH), 4.44-4.51 (dd, 1H, J=4.4, 17.2Hz, CH), 3.84 (s, 2H, CH 2 ), 2.43 (brs, 2H, CH 2 ), 2.01-2.26 (m, 2H, CH 2 ).
Embodiment 3
[0038]Take compound GY005 (0.21g, 0.5mmol), add methanol (20mL) to dissolve at room temperature, then add solid potassium hydroxide (65mg, 1.2mmol), stir at room temperature for 2h, concentrate to dryness, wash with cold ethanol twice, and dry in vacuo GY004 was obtained as a white powdery solid (0.23 g, 92%). HRMS(M+H)415.7743; 1 H-NMR (D 2 O, 400Hz), δ: 7.87 (d, 1H, J = 8.4Hz, CH), 7.64 (d, 1H, J = 7.2Hz, CH), 6.50-6.54 (dd, 1H, J = 5.2, 5.6Hz, CH), 6.08(d, 1H, J=8.0Hz, CH), 5.19-5.35(ddd, 1H, J=5.2, 4.8, 43.6Hz, CH), 4.48-4.54(m, 1H, CH), 3.93- 3.94 (m, 2H, CH 2 ), 2.27-2.31 (m, 2H, CH 2 ), 1.94-2.15 (m, 2H, CH 2 ).
[0039] The above are only part of the embodiments of the present invention, and are not intended to limit the present invention in any form. Any simple modifications made to the above embodiments according to the technical essence of the present invention, equivalent changes and modifications, all belong to this invention. within the scope of the techni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com