Benzoyl spirocyclic aromatic hydrocarbon sterically hindered luminescent material and preparation method thereof
A technology of benzoyl spiro aromatic hydrocarbons and luminescent materials, applied in luminescent materials, chemical instruments and methods, and condensation preparation of carbonyl compounds, etc., can solve the problems that materials cannot be fully considered, and achieve large steric hindrance effect and good thermal stability sex, mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
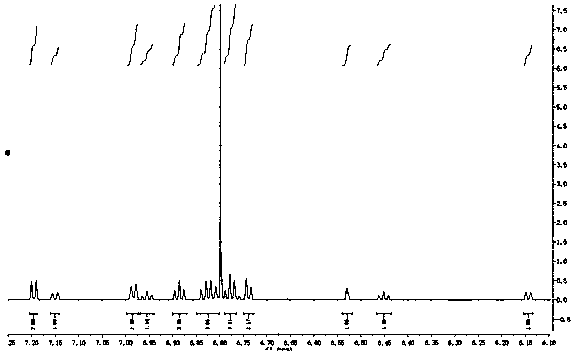
[0055] Example 1: 2'-[benzoyl]-spiro-9,9'-xanthene fluorene
[0056] Dissolve spiro-9,9'-xanthene fluorene (3.310g, 10.0mmol), anhydrous aluminum trichloride (1.602g, 12.0mmol) in dichloromethane solution, and benzoyl chloride (1.6800g, 12.0mmol) was added dropwise to the reaction flask, and reacted at room temperature for 12 hours. After completion of the reaction, wash with saturated sodium carbonate solution, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase, and obtain the target product with a mixed solvent column chromatography of ethyl acetate and sherwood oil, (yield: 78 %), LC-MS (EI) m / z437.1555 [M + ].
Embodiment 2
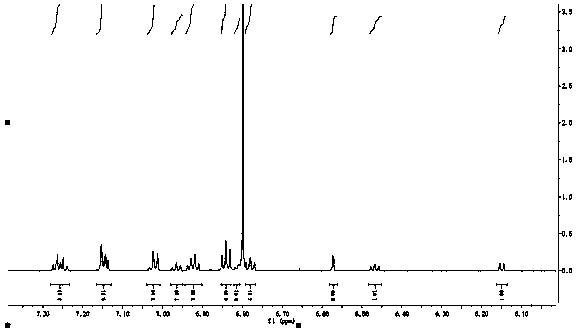
[0057] Example 2: 2,2'-bis[benzoyl]-spiro-9,9'-xanthene fluorene
[0058] Dissolve spiro-9,9'-xanthene fluorene (3.310g, 10.0mmol), anhydrous aluminum trichloride (3.2040g, 24.0mmol) in dichloromethane solution, and benzoyl chloride (3.3600g, 24.0mmol) was added dropwise to the reaction flask, and reacted at room temperature for 12 hours. Wash with saturated sodium carbonate solution after completion of the reaction, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase, obtain the target product with a mixed solvent column chromatography of ethyl acetate and sherwood oil, (yield: 71 %).
Embodiment 3
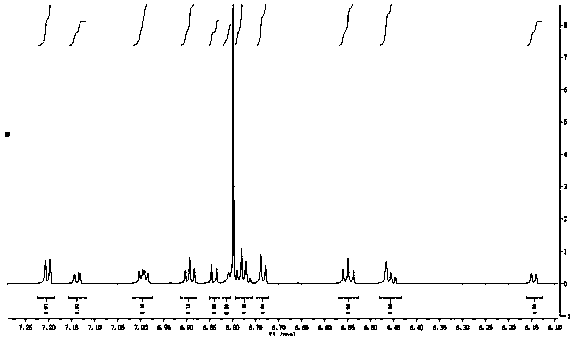
[0059] Example 3: 2'-[p-fluorobenzoyl]-spiro-9,9'-xanthene fluorene
[0060] Dissolve spiro-9,9'-xanthene fluorene (3.310g, 10.0mmol), anhydrous aluminum trichloride (1.602g, 12.0mmol) in dichloromethane solution, and p-fluorobenzoyl chloride (1.9027 g, 12.0mmol) was added dropwise to the reaction flask, and reacted at room temperature for 12 hours. After completion of the reaction, wash with saturated sodium carbonate solution, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase, and obtain the target product with a mixed solvent column chromatography of ethyl acetate and sherwood oil, (yield: 78 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com