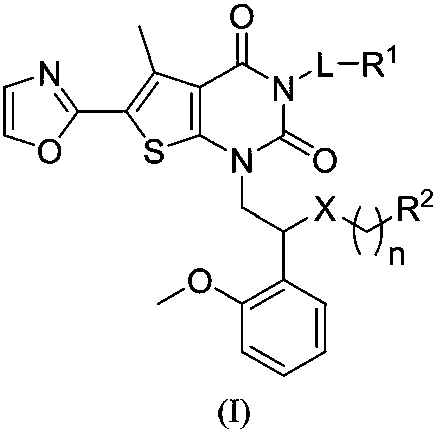
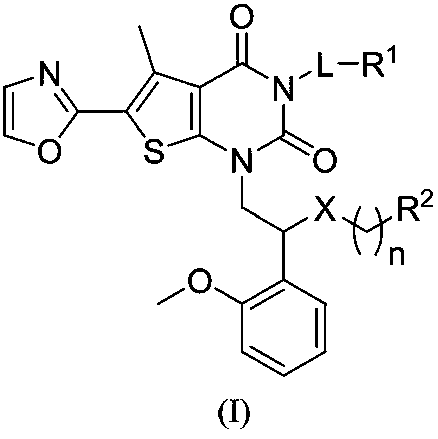
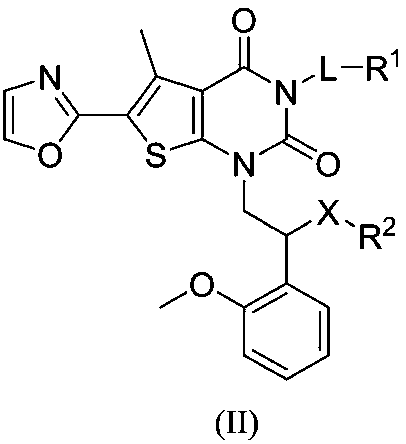
Compound used as ACC (Acetyl CoA Carboxylase) inhibitor and application thereof
A technology of compounds and solvates, applied to compounds as ACC inhibitors and their application fields, can solve the problems of lack of therapeutic strategies and limited therapeutic effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1: 3-(1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl- 6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)cyclobutane-1-carboxylic acid
[0113]
[0114] Step 1: Preparation of ethyl 3-oxocyclobutanecarboxylate
[0115]
[0116] In a 1000mL single-necked bottle, dissolve 3-oxocyclobutanecarboxylic acid (25.0g, 219.1mmol) in toluene (500mL), add triethyl orthoacetate (106.6g, 657.3mmol), and heat at 110°C Stir for 5h. After the reaction was completed, it was cooled to room temperature, and dilute hydrochloric acid (1.0M, 20 mL) was added to quench the reaction. The organic layer was separated, washed once with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 24.9 g of the title compound, which was directly used in the next reaction without purification.
[0117] Step 2: Preparati...
Embodiment 2
[0141] Example 2: 3-(3,3-difluorocyclobutyl)-1-(2-(2-methoxyphenyl)-2–((tetrahydro-2H-pyran-4-yl)oxy Base) ethyl)-5-methyl-6-(oxazol-2-yl)thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione
[0142]
[0143] Step 1: Preparation of ethyl 2-(3-(3,3-difluorocyclobutylamino)ureido)-4-methyl-thiophene-3-carboxylate
[0144]
[0145] In a low-temperature bath at -5°C, dissolve ethyl 2-amino-4-methylthiophene-3-carboxylate (5.786g, 19.5mmol) and triethylamine (11.84g, 117mmol) in anhydrous dichloromethane (110mL) The solution was added dropwise to a solution of triphosgene (5.786 g, 19.5 mmol) in dry dichloromethane (50 mL). After dripping, stir at 0°C for 1.5h and move to room temperature. Add 3,3-difluorocyclobutylamine (2.8 g, 19.5 mmol) and react overnight. Concentration, silica gel column chromatography, and then beating with ethyl acetate, the product was dissolved in ethyl acetate. The mother liquor was concentrated to obtain 4.5 g of the title compound, with a yield of 80%. M...
Embodiment 3
[0158] Example 3: (1R,3r)-3-(1-((R)-2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy )ethyl)-5-methyl-6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidine-3(2H) Preparation of -yl)-1-methylcyclobutanecarboxylic acid
[0159]
[0160] Step 1: Preparation of methyl 3-(dibenzylamino)-1-methylcyclobutanecarboxylate
[0161]
[0162] In a 1000mL single-necked bottle, dissolve methyl 1-methyl-3-oxo-cyclobutanecarboxylate (4.95g, 34.8mmol) in anhydrous tetrahydrofuran (200mL), add glacial acetic acid (22mL), dibenzylamine (7.56g, 38.3mmol), sodium triacetoxyborohydride (14.8g, 69.7mmol), and stirred overnight at room temperature. After the reaction, filter, concentrate the filtrate, add dichloromethane (300mL) to dissolve, wash with water, saturated aqueous sodium bicarbonate, and saturated aqueous sodium chloride in turn, dry the organic layer over anhydrous sodium sulfate, filter, concentrate the filtrate, and place on a silica gel column Chromatography (petrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com